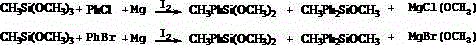Method for synthesizing phenyl-containing organosilicon monomers
A synthesis method and organosilicon technology, applied in the direction of organic chemistry, chemical instruments and methods, compounds of group 4/14 elements of the periodic table, etc., can solve the problems of high production cost and low yield, achieve fast reaction speed, The effect of high activity and excellent application performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 150 parts of toluene, 32 parts of methyltrimethoxysilane, 4 parts of magnesium, 5 parts of chlorobenzene, and 0.01 part of iodine into the reaction kettle in sequence, and heat to 80°C for activation and initiation reaction. After activation and initiation reaction for 1 hour, then Raise the temperature to 120°C, and gradually add 55 parts of chlorobenzene. The reaction is complete. Filter and distill to obtain the product methylphenyldimethoxysilane, methyldiphenylmethoxysilane.
[0033] The selectivity of methylphenyldimethoxysilane is 75%, and the selectivity of methyldiphenylmethoxysilane is 5%.
Embodiment 2
[0035] Add 245 parts of 2-methyltetrahydrofuran, 42 parts of methyltrimethoxysilane, 6 parts of magnesium, 8 parts of chlorobenzene, and 0.05 parts of iodine to the reaction kettle in sequence, and heat to 40°C to initiate the activation reaction. After hours, the temperature was raised to 60°C, and 42 parts of chlorobenzene were gradually added. The reaction is complete. Filter and distill to obtain the product methylphenyldimethoxysilane, methyldiphenylmethoxysilane.
[0036] The selectivity of methylphenyldimethoxysilane is 81%, and the selectivity of methyldiphenylmethoxysilane is 4%.
Embodiment 3
[0038] Add 250 parts of toluene, 100 parts of 2-methyltetrahydrofuran, 48 parts of methyltrimethoxysilane, 7 parts of magnesium, 8 parts of chlorobenzene, and 0.1 part of iodine to the reactor in sequence, and heat to 60°C for activation to initiate the reaction. After initiating the reaction for 1.5 hours, the temperature was raised to 110° C., and 40 parts of chlorobenzene were gradually added. The reaction is complete. Filter and distill to obtain the product methylphenyldimethoxysilane, methyldiphenylmethoxysilane.
[0039] The selectivity of methylphenyldimethoxysilane is 85%, and the selectivity of methyldiphenylmethoxysilane is 3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com