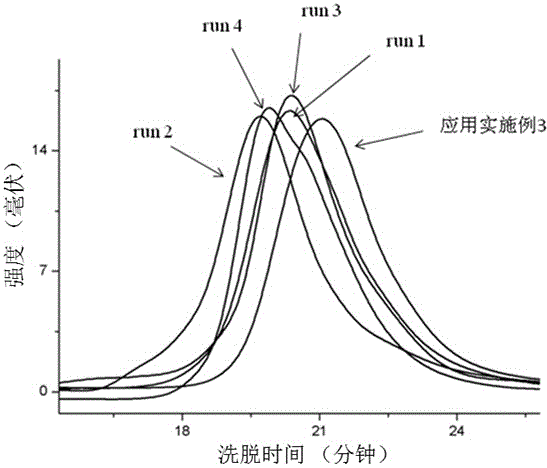
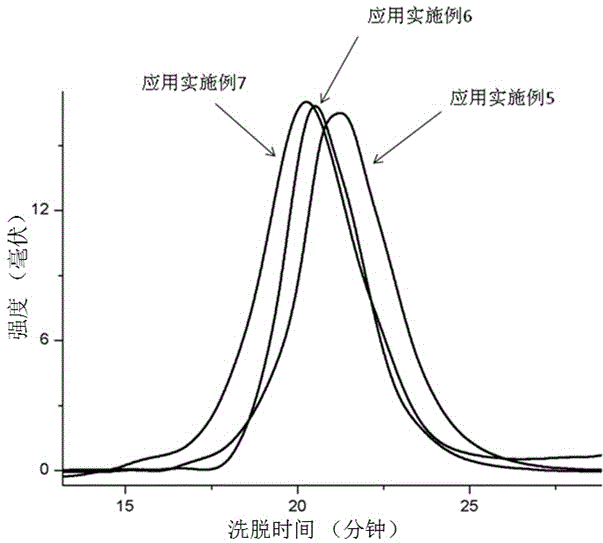
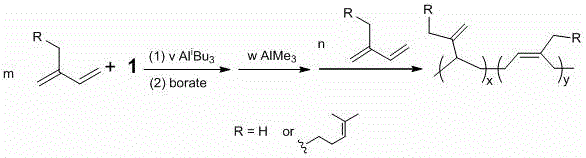
Method for preparing regioblock copolymers of isoprene and myrcene by chain transfer reaction
A technology of isoprene and myrcene, which is applied in the field of catalyzing the polymerization of isoprene or myrcene to form di- or multi-block regional copolymers, which can solve the disadvantages of environmental protection and energy saving, and no report on isoprene regional copolymers , increase production costs and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Weigh 0.0155 g (0.01 mmol) of complex 1a into a 100 mL eggplant-shaped bottle, dissolve it with 18 mL of chlorobenzene, then weigh 0.511 g (7.5 mmol) of isoprene, and inject 100 μL of 1M Al i Bu 3 The n-hexane solution was injected into the above mixture. Weigh 0.0185 g (0.02 mmol) [Ph3C][B(C6F5)4] (cocatalyst borate), add 2mL of chlorobenzene to dissolve, and transfer to the dropping funnel. Assemble the dropping funnel and the eggplant-shaped bottle, start stirring, and quickly add the co-catalyst into the solution after constant temperature at 25 degrees Celsius. After 10 min, add 100 μL of 1 M AlMe 3 The n-heptane solution was stirred for 2 minutes, then 0.511 g (7.5 mmol) of isoprene was added and reacted for 45 minutes. After the reaction, ethanol was slowly added dropwise with stirring until the solid was completely precipitated. The liquid was decanted and the 3,4 and 1,4-polyisoprene copolymers were obtained as white solids. Dry in a vacuum oven at 60°C t...
Embodiment 2
[0048] Weigh 0.0156 g (0.01 mmol) of complex 1b into a 100 mL eggplant-shaped bottle, dissolve it with 18 mL of chlorobenzene, then weigh 0.511 g (7.5 mmol) of isoprene, add 100 μL of 1M Al i Bu 3 The n-hexane solution was injected into the above mixture. Weigh 0.0185 g (0.02 mmol) [Ph3C][B(C6F5)4] (cocatalyst borate), add 2mL of chlorobenzene to dissolve, and transfer to the dropping funnel. Assemble the dropping funnel and the eggplant-shaped bottle, start stirring, and quickly add the co-catalyst into the solution after constant temperature at 25 degrees Celsius. After 10 min, add 100 μL of 1 M AlMe 3 The n-heptane solution was stirred for 2 minutes, then 0.511 g (7.5 mmol) of isoprene was added and reacted for 45 minutes. After the reaction, ethanol was slowly added dropwise with stirring until the solid was completely precipitated. The liquid was decanted and the 3,4 and 1,4-polyisoprene copolymers were obtained as white solids. Drying in a vacuum oven at 60°C until...
Embodiment 3
[0050] Weigh 0.0156 g (0.01 mmol) of complex 1b into a 100 mL eggplant-shaped flask, dissolve it with 18 mL of chlorobenzene, then weigh 0.511 g (7.5 mmol) of isoprene, add 100 μL of Al i Bu 3 The n-hexane solution was injected into the above mixture. Weigh 0.0185 g (0.02 mmol) [Ph3C][B(C6F5)4] (cocatalyst borate), add 2 mL of chlorobenzene to dissolve, and transfer to the dropping funnel. Assemble the dropping funnel and the eggplant-shaped bottle, start stirring, and quickly add the co-catalyst into the solution after constant temperature at 25 degrees Celsius. After 10 min, add 140 μL of 1M AlMe 3 The n-heptane solution was stirred for 2 minutes, then 0.511 g (7.5 mmol) of isoprene was added and reacted for 45 minutes. After the reaction, ethanol was slowly added dropwise with stirring until the solid was completely precipitated. The liquid was decanted and the 3,4 and 1,4-polyisoprene copolymers were obtained as white solids. After drying in a vacuum oven at 60°C to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com