Method for preparing borneol
A technology of orthoborneol and borneol, which is applied in the field of preparing orthoborneol, can solve problems such as low yield, complicated reaction, and low cost, and achieve the effects of high product yield, simple post-processing, and reduced cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
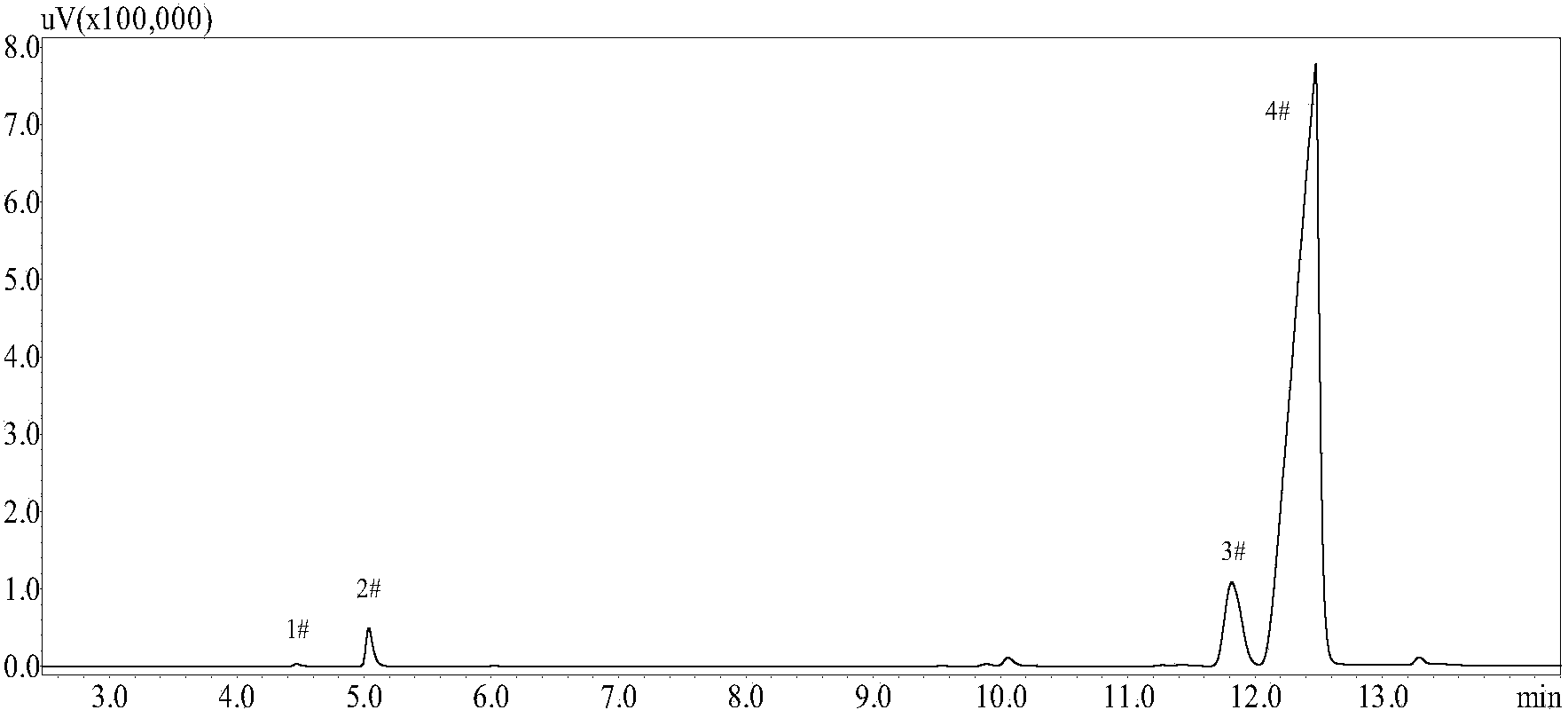
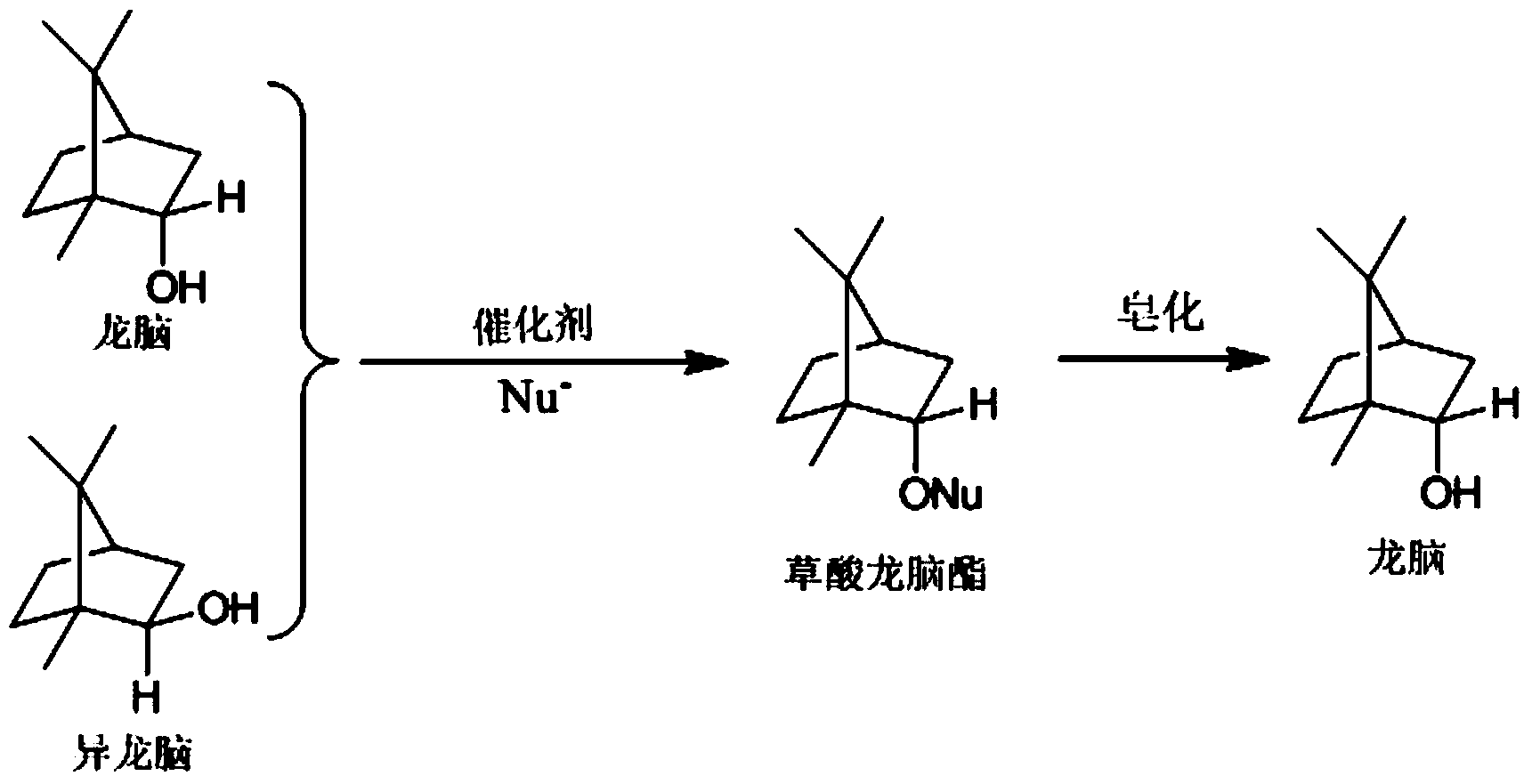
[0031]The method for preparing orthoborneol of the present invention comprises the following steps: dissolving the synthesized crude borneol in an organic solvent, and then standing for a certain period of time to remove impurities such as moisture and soil in the lower layer. Add boric anhydride, pyroboric acid, metaboric acid or metatitanic acid catalyst, react with a certain amount of anhydrous oxalic acid at a certain temperature for a period of time, filter to remove unreacted anhydrous oxalic acid and catalyst after the reaction, wash 3 to 5 times with water The catalyst and oxalic acid are further removed. After the organic solution is distilled to recover the solvent, tricyclene, camphene and a small amount of borneol and isoborneol are removed by steam distillation to obtain bornyl oxalate and isobornyl oxalate. The obtained bornyl oxalate and isobornyl oxalate are subjected to saponification to obtain a high content borneo product. This reaction can use aromatic hyd...
Embodiment 1
[0035] In a 250mL four-necked round-bottomed flask, add 10.0g of crude borneol (commercially available borneol, containing 60.6%wt of borneol), add 1.0g of boric anhydride catalyst and 2.5g of metatitanic acid catalyst, 15.0g of anhydrous oxalic acid, Use 30.0g of n-hexane as solvent, stir and condense to reflux, maintain the temperature at 54-56°C, and the reaction time is 24h. After the reaction is completed, remove the solvent, camphene and tricyclene, and saponify the remaining liquid to obtain a crude product. After refining, 3.1g The orthoborneol product has a content of 88.1% (orthoborneol:isoborneol=88.1:8.1), and the orthoborneol extraction rate is 45.1%.
Embodiment 2
[0037] In a 250mL four-necked round-bottomed flask, add 5.0g of crude borneol (containing 54.2%wt of borneol), add 1.0g of boric anhydride catalyst, 5.5g of anhydrous oxalic acid, and use 65.7g of n-hexane as a solvent, stir and condense to reflux , temperature is maintained at 54~56 ℃, and reaction time 35h, after reaction finishes, remove solvent and amphene, tricyclic olefin, remaining liquid saponification obtains crude product after refining and obtains 2.1g orthoborneol product, and content is 71.8% (orthoborneol Brain:isoborneol=71.8:12.3), the extraction rate of orthoborneol was 55.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com