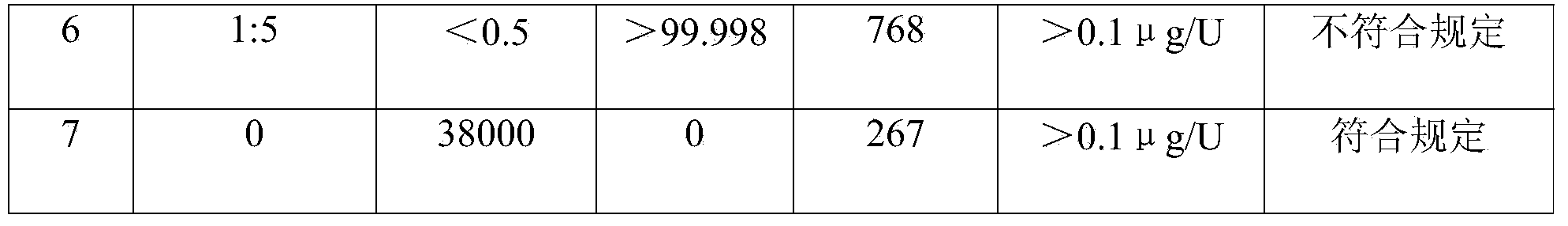Method for removing endotoxin out of polypeptide
An endotoxin and corticotropin technology, which is applied in the preparation methods of peptides, chemical instruments and methods, biochemical equipment and methods, etc., can solve the problems of long time consumption, inactivation of polypeptides, and inability to completely remove them, so as to improve production efficiency. , the effect of reducing the operating volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
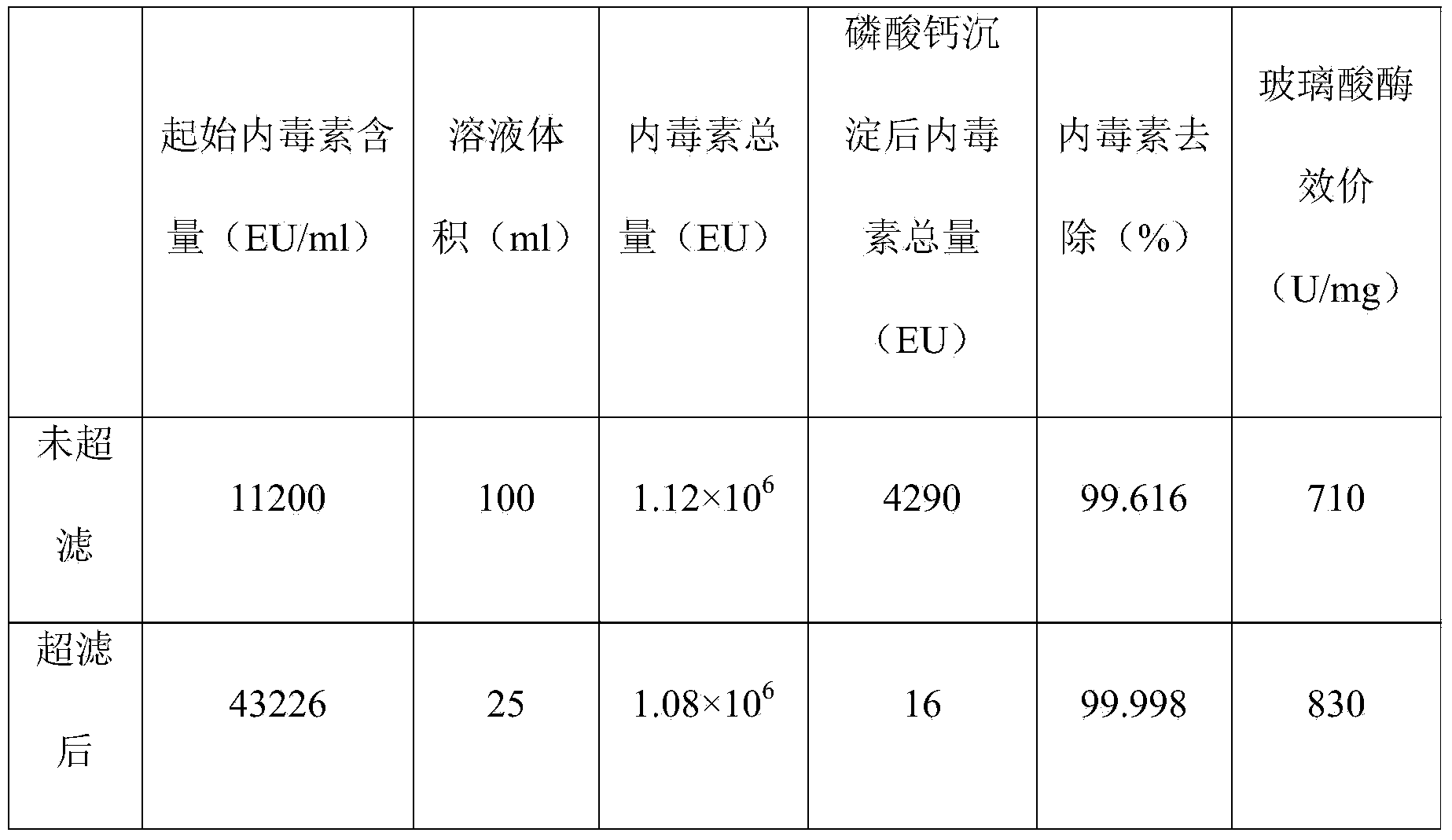
[0038] The crude hyaluronidase 1220g, batch number 090122, was purchased from Lixin Biochemical Products Factory in Tianshan District, Urumqi. The crude hyaluronidase was obtained from fresh bovine testis by separating, mincing into pulp, brewing in acid solution, pulling pulp, and salting out. Below 250U / mg. Above-mentioned crude product is put into stainless steel barrel, according to raw material (total titer): purified water (volume)=100 million U: 800ml dissolve, namely add 4 ℃ of purified water 2800ml, stir for 1 hour to completely dissolve, the solution after dissolving is filtered, filtrate is used A 10kD ultrafiltration membrane (model: Hydrosart, Germany Sartorius) was used for ultrafiltration concentration, and the solution volume after ultrafiltration was 530ml. Slowly add 60ml 0.4mol / L 4°C sodium phosphate solution, stir, then slowly add 36ml 1mol / L 4°C calcium acetate solution, stir, adjust pH to 8.40 with 2.5M NaOH, stir for 10 minutes and freeze at 3000 rpm Ce...
Embodiment 2
[0044]Crude hyaluronidase 1400g, batch number 090123, purchased from Lixin Biochemical Products Factory in Tianshan District, Urumqi. The crude hyaluronidase is obtained from fresh bovine testis by separating, mincing into pulp, brewing in acid solution, pulling pulp, and salting out. Below 250U / mg. The above-mentioned crude product was put into a stainless steel barrel, and dissolved according to the raw material (total titer): purified water (volume)=100 million U: 800ml, namely, 3300ml of purified water at 4° C. was added, stirred for 1 hour to completely dissolve, the dissolved solution was filtered, and the filtrate was used A 5kD ultrafiltration membrane (model: Hydrosart, Germany Sartorius) was used for ultrafiltration concentration, and the solution volume after ultrafiltration was 650 ml. Slowly add 70ml 0.4mol / L 4°C sodium phosphate solution, stir, then slowly add 42ml 1mol / L 4°C calcium acetate solution, stir, adjust pH to 8.60 with 2.5M NaOH, stir for 10 minutes an...
Embodiment 3
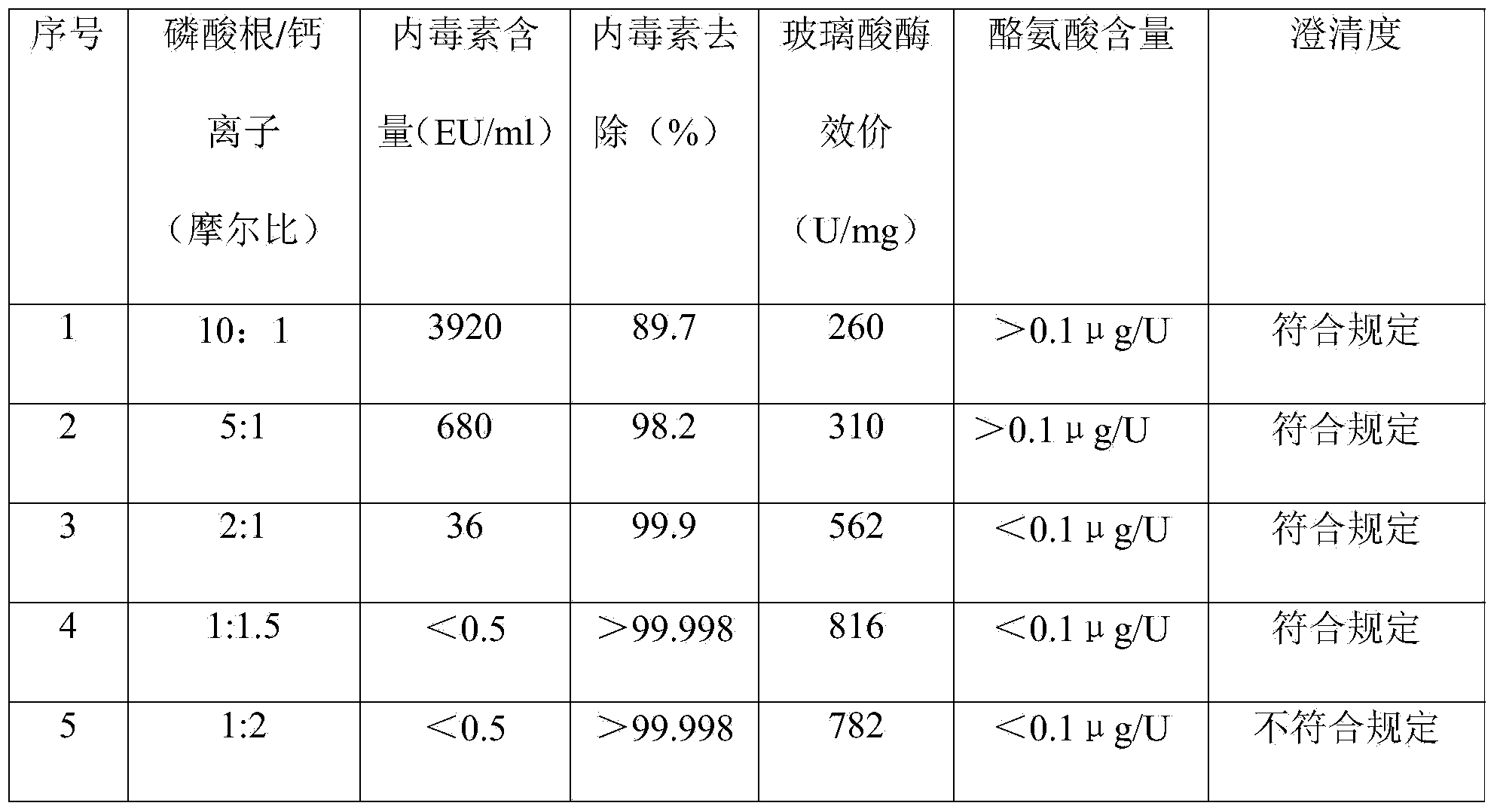
[0050] The crude hyaluronidase batch No. 100501 was purchased from Lixin Biochemical Products Factory in Tianshan District, Urumu. The crude hyaluronidase was obtained from fresh bovine testis by separating, mincing into pulp, soaking in acid liquid, pulling pulp, and salting out. Its specific activity should not be lower than 250U / mg; 500g of hyaluronidase crude product was charged, and dissolved according to raw material (total titer): purified water (volume) = 100 million U: 800ml, that is, 1120ml of purified water at 4°C was added, and stirred for 1 hour to completely dissolve. The solution was filtered, 100 ml of samples were reserved for testing, and the rest were subjected to one-step ultrafiltration to obtain an ultrafiltrate (refer to Example 1 for specific operations). The ultrafiltrate is evenly divided into several parts, and sodium phosphate and calcium acetate solutions of 0.1 times the volume of ultrafiltrate are added in turn to achieve the final molar ratio of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com