Method for preparing tributyrin
A technology of tributyrin, glycerol, applied in the preparation of carboxylic acid halide, organic chemistry and other directions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
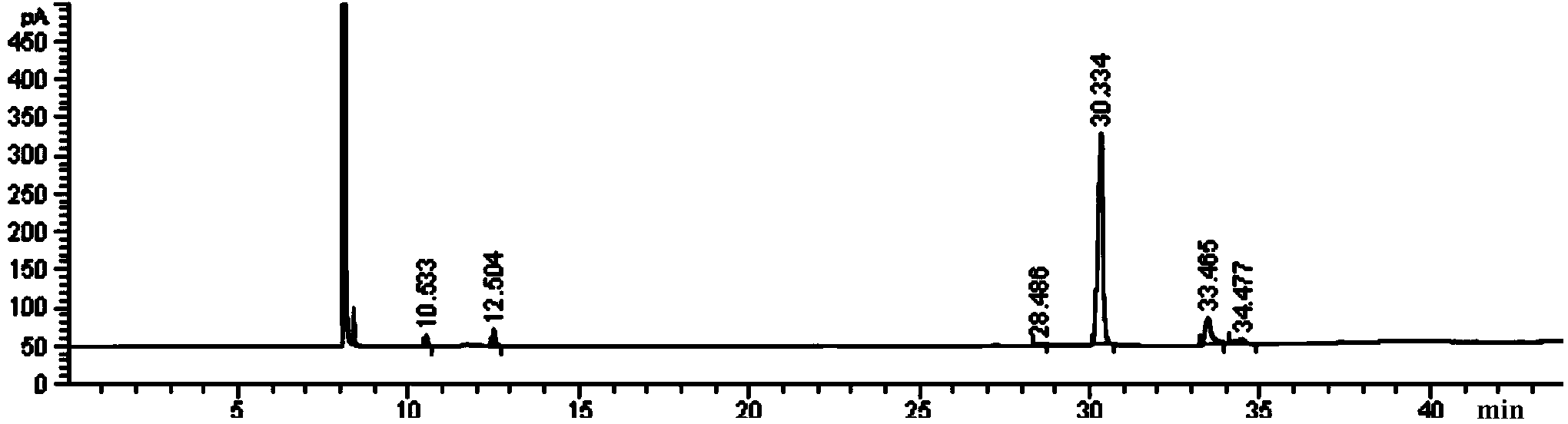
[0026] Put 8.6ml of glycerin, 55ml of triethylamine and 250ml of chloroform into a 500ml three-necked flask, and then add 37.5ml of butyryl chloride dropwise into the reaction flask under the conditions of ice bath and magnetic stirring for 3 hours. After dropping, continue to react for 0.5h, then remove the ice-water bath, and finish the reaction at room temperature for 2.5h. The reacted solid-liquid mixture was extracted five times with water, each time using 250 ml of water. After the aqueous phases were combined, the pH was adjusted to 10 with NaOH solution, and the free triethylamine was dried with anhydrous sodium sulfate and recycled. The organic phase after washing with water was dried with anhydrous sodium sulfate for 2 h, and then the chloroform was evaporated under reduced pressure to obtain 39.9 g of the product, and the content of tributyrin in the product was 80.25% (GC).
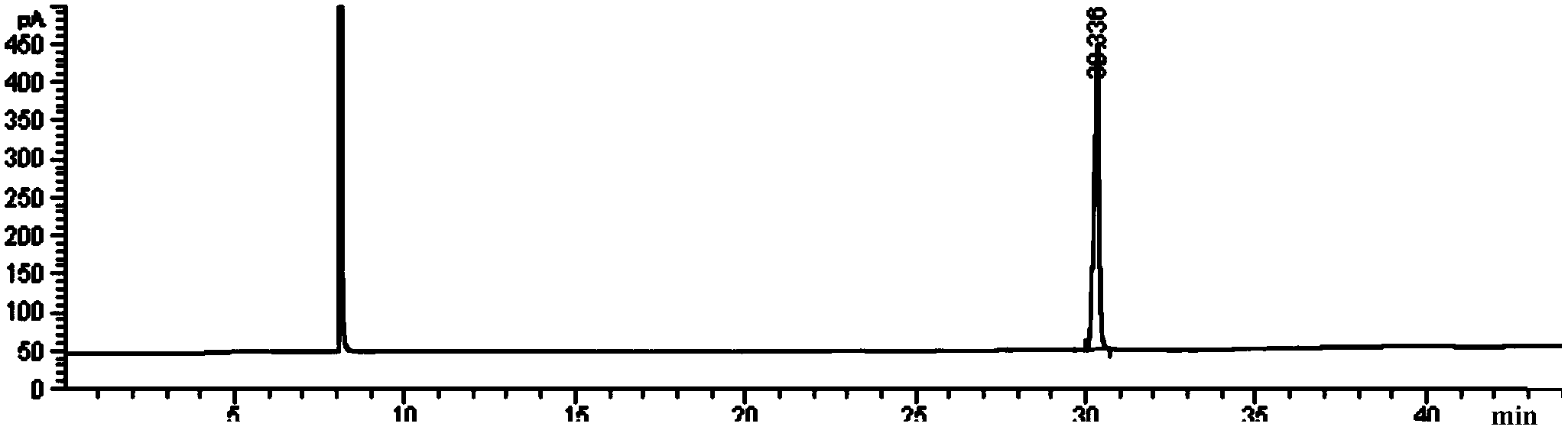
[0027] figure 1 It is the gas chromatography (GC) figure of tributyrin reference substan...
Embodiment 2
[0031] Put 5.2ml of glycerin, 33ml of triethylamine and 150ml of chloroform into a 500ml three-necked flask, and then add 24.75ml of butyryl chloride dropwise into the reaction flask under the conditions of ice-water bath and magnetic stirring, and the dropping time is 2h. After dropping, the reaction was continued for 1 h, and then the ice-water bath was removed, and the reaction was completed at room temperature for 3 h. The reacted solid-liquid mixture was extracted five times with water, each time using 200 ml of water. After the aqueous phases were combined, the pH was adjusted to 10 with NaOH solution, and the free triethylamine was dried with anhydrous sodium sulfate and recycled. The organic phase after washing with water was dried with anhydrous sodium sulfate for 2 h, and then the chloroform was evaporated under reduced pressure to obtain 24.4 g of the product, and the content of tributyrin in the product was 83.76% (GC).
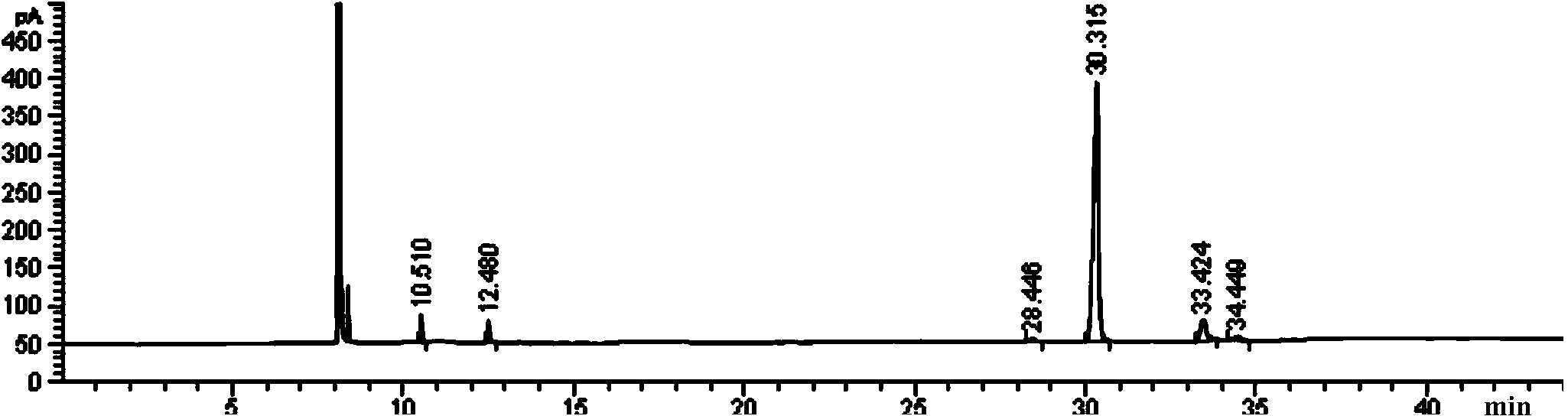
[0032] image 3 It is the gas chromatogra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com