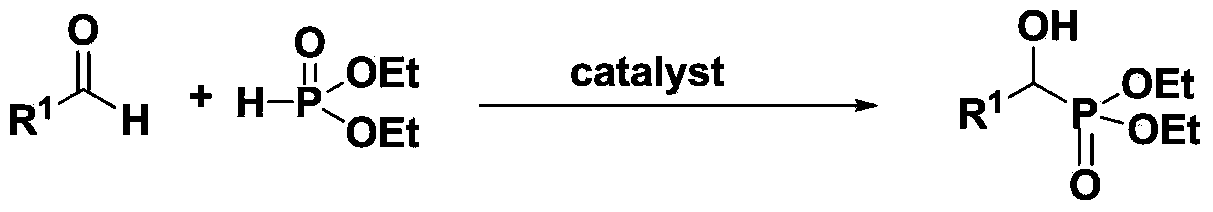
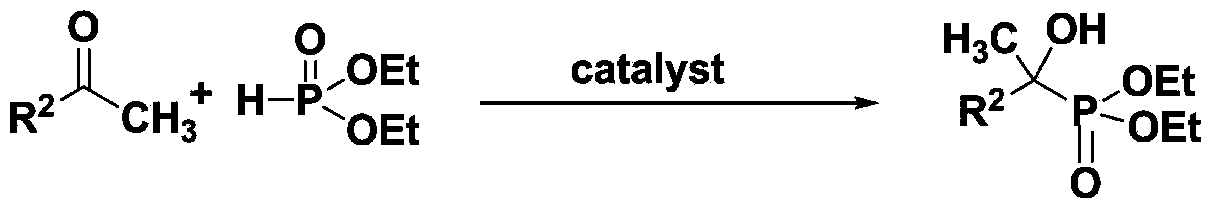
Method for preparing hydroxyl phosphonate
A technology of hydroxyphosphonate and hydrogen phosphine, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., to achieve high yield, mild reaction conditions, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
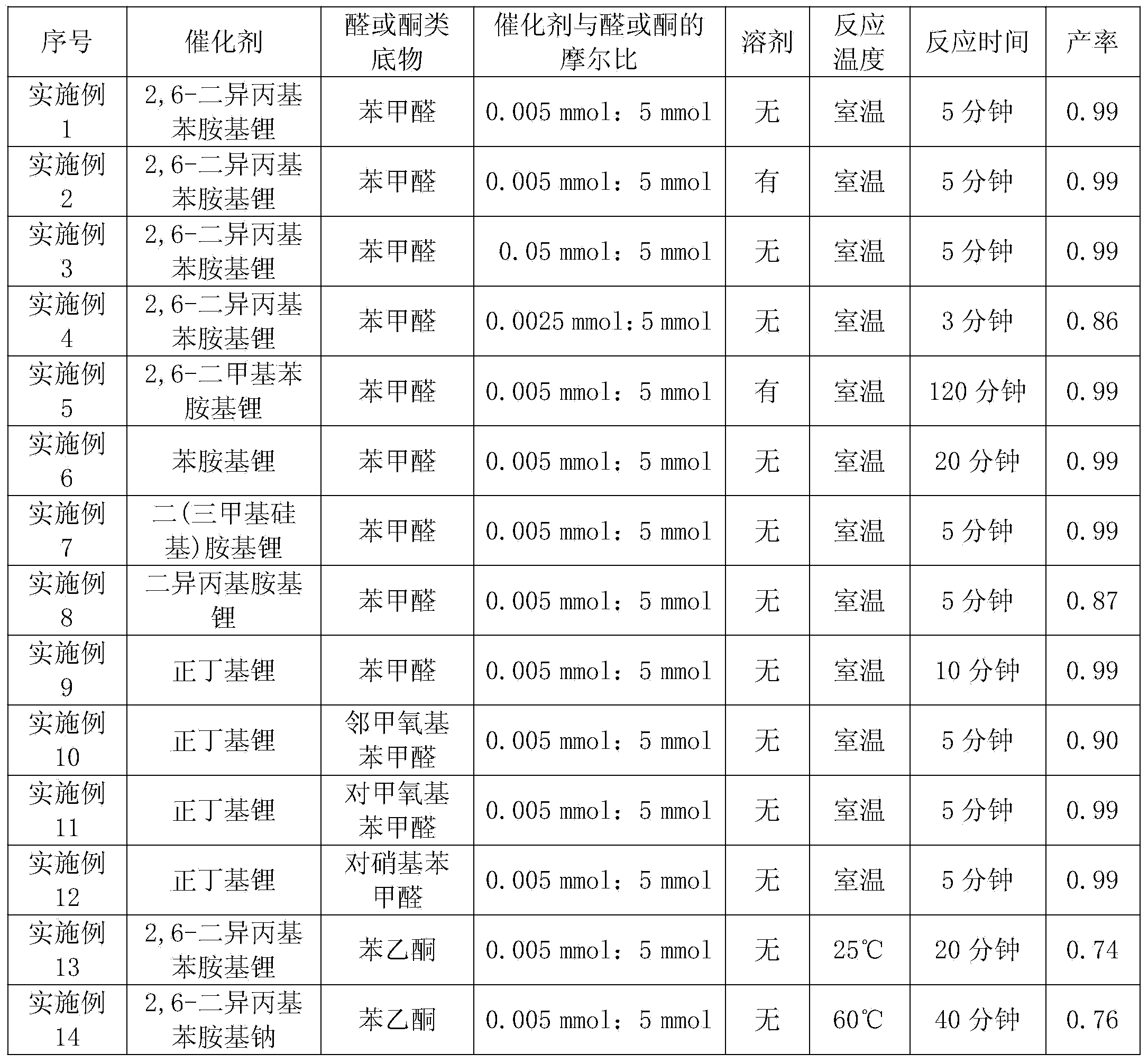
[0033] Example 1: 2,6-Diisopropylanilinide lithium catalyzes the reaction of benzaldehyde and diethyl phosphite
[0034] Under anhydrous, oxygen-free and argon protection, diethyl phosphite (0.77mL, 6mmol) and 2,6-diisopropylanilinidelithium (0.005mmol) were successively added to a 30mL Schlenk reaction flask, and at room temperature After stirring for 10 minutes, benzaldehyde (0.51 mL, 5 mmol) was added, and after reacting at room temperature for 5 minutes, deionized water was added to terminate the reaction. Extracted with ethyl acetate (10mL×3), dried overnight with anhydrous sodium sulfate, filtered, spin-dried, washed with n-hexane to obtain a white solid, dried to constant weight to obtain 1.21 grams of α-hydroxyphosphonate, the calculated yield was 99%.
[0035] The nuclear magnetic analysis for this α-hydroxy phosphonate is as follows: 1 H NMR (CDCl 3 ,400MHz,ppm)δ7.50-7.48(2H,m,ArH),7.37-7.28(3H,m,ArH),5.03-5.00(1H,d,J=10.8Hz,CH),4.08-3.94(4H ,m,CH 2 ), 3.62 (1H,...
Embodiment 2
[0036] Example 2: 2,6-diisopropylanilinide lithium catalyzes the reaction of benzaldehyde and diethyl phosphite
[0037] Under anhydrous, oxygen-free and argon protection, diethyl phosphite (0.77 mL, 6 mmol), 2,6-diisopropylanilinide lithium (0.005 mmol), n-hexane ( 2 mL), stirred at room temperature for 10 minutes, then added benzaldehyde (0.51 mL, 5 mmol), and reacted at room temperature for 5 minutes, then added deionized water to terminate the reaction. Extracted with ethyl acetate (10mL×3), dried overnight with anhydrous sodium sulfate, filtered, spin-dried, washed with n-hexane to obtain a white solid, dried to constant weight to obtain 1.21 grams of α-hydroxyphosphonate, the calculated yield was 99%.
Embodiment 3
[0038] Example Three: 2,6-Diisopropylanilinide Lithium Catalyzed Reaction of Benzaldehyde and Diethyl Phosphite
[0039] Under anhydrous, oxygen-free and argon protection, diethyl phosphite (0.77mL, 6mmol) and 2,6-diisopropylanilinidelithium (0.05mmol) were successively added to a 30mL Schlenk reaction flask, and After stirring for 10 minutes, benzaldehyde (0.51 mL, 5 mmol) was added, and after reacting at room temperature for 5 minutes, deionized water was added to terminate the reaction. Extracted with ethyl acetate (10mL×3), dried overnight with anhydrous sodium sulfate, filtered, spin-dried, washed with n-hexane to obtain a white solid, dried to constant weight to obtain 1.21 grams of α-hydroxyphosphonate, the calculated yield was 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com