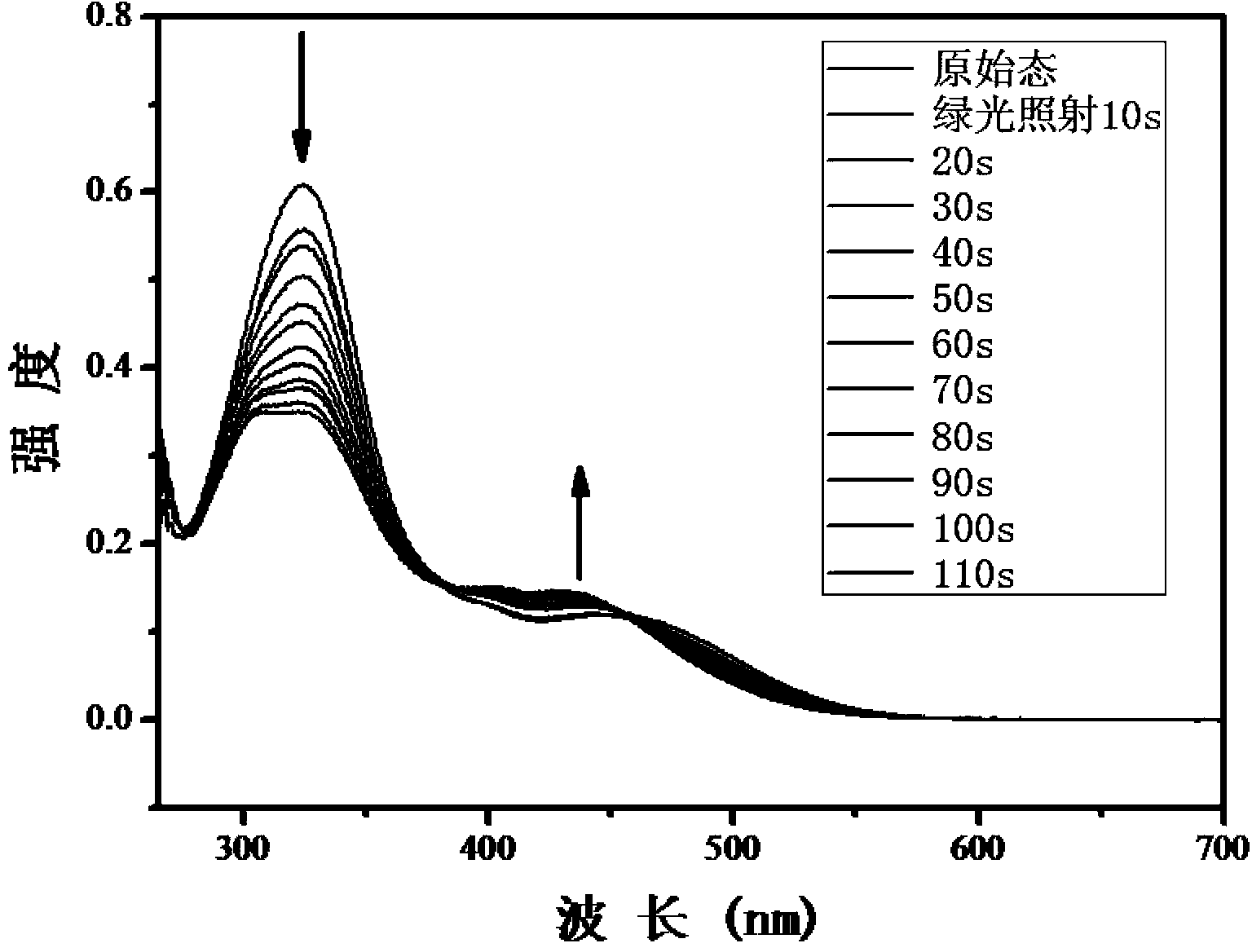
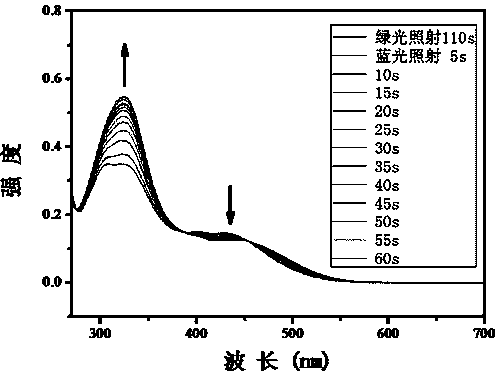
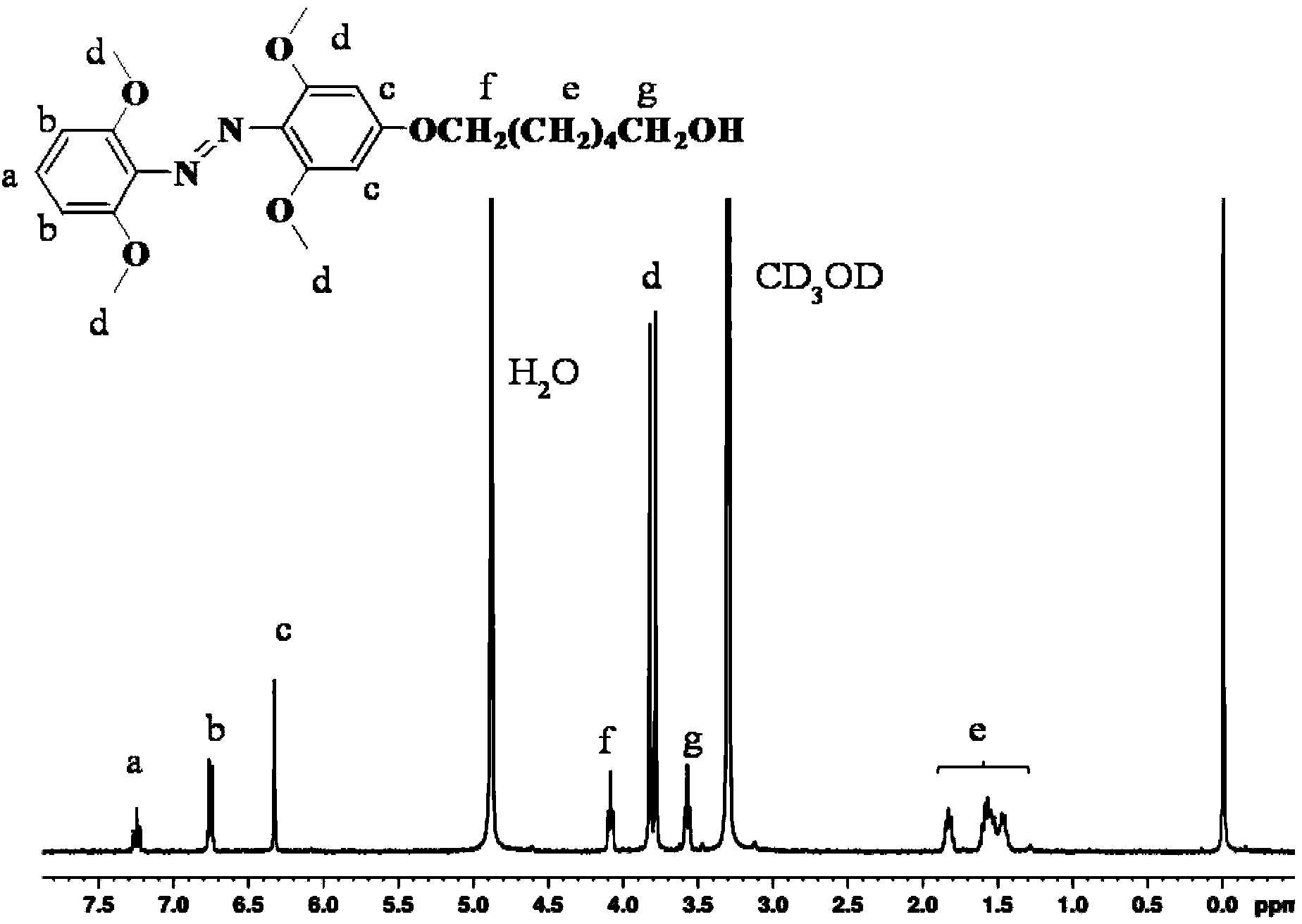
Method for synthesizing visible-light response type azobenzene polymer
A synthetic method, azobenzene technology, applied in the field of synthesizing visible light-responsive azobenzene polymers, can solve problems such as limited applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of 2,6,2'6'-tetramethoxyazophenol: Dimethoxyaniline (1.53g, 0.01mol), concentrated hydrochloric acid (36%, 2.54ml) and deionized water (3ml) were mixed, Make it into a paste, and control the temperature at about 0°C. Slowly add NaNO dropwise with stirring 2 (0.015mol, 1.035g) 10mL cold aqueous solution. Stir the reaction until the solution makes the starch KI turn blue, filter to obtain the diazonium salt solution; dimethoxyphenol (1.54g, 0.01mol) is dissolved in NaOH (1g, 0.025mol) aqueous solution (10ml); the aforementioned diazonium salt The solution was added dropwise to the phenol solution, and during the dropwise addition, saturated Na 2 CO 3 The solution was adjusted to maintain a pH of 10. After the dropwise addition, continue to stir for 12 h, neutralize with dilute HCl to pH = 6, and stir for 10 min. Suction filtration, the product was washed with deionized water until the filtrate was neutral, and dried.
[0027] Preparation of 2,6,2'6'-tetr...
Embodiment 2
[0031] Preparation of 2,6,2',6'-tetramethoxyazophenol: Dimethoxyaniline (1.53g, 0.01mol), concentrated hydrochloric acid (36%, 3ml) and deionized water (3.5ml) were mixed , into a paste, and the temperature is controlled at about 0°C. Slowly add NaNO dropwise with stirring 2 (0.023mol, 1.55g) 15mL cold aqueous solution. Stir the reaction until the solution makes starch KI turn blue, filter to obtain a diazonium salt solution; dimethoxyphenol (1.54g, 0.01mol) is dissolved in NaOH (1.5g, 0.038mol) aqueous solution (15ml); the aforementioned diazonium The salt solution was added dropwise to the phenol solution. During the dropwise addition, saturated Na 2 CO 3 The solution was adjusted to maintain a pH of 10. After the dropwise addition, continue to stir for 12 h, neutralize with dilute HCl to pH = 6, and stir for 10 min. Suction filtration, the product was washed with deionized water until the filtrate was neutral, and dried.
[0032] Preparation of 2,6,2',6'-tetramethoxy-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com