Vaccine composition for preventing and treating respiratory diseases secondary to atrophic rhinitis, and preparation method and application thereof
A technology for respiratory diseases and vaccine compositions, which is applied in the field of vaccine compositions for respiratory diseases, and can solve problems such as difficulty in achieving immune effects, high cost, and short protection period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
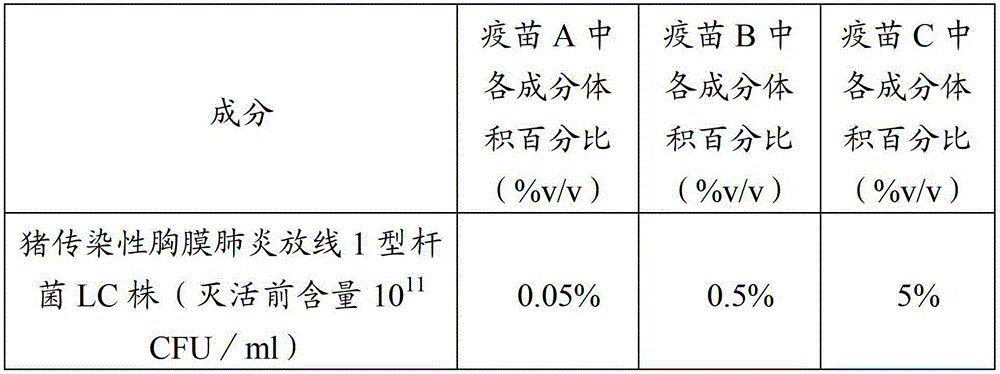
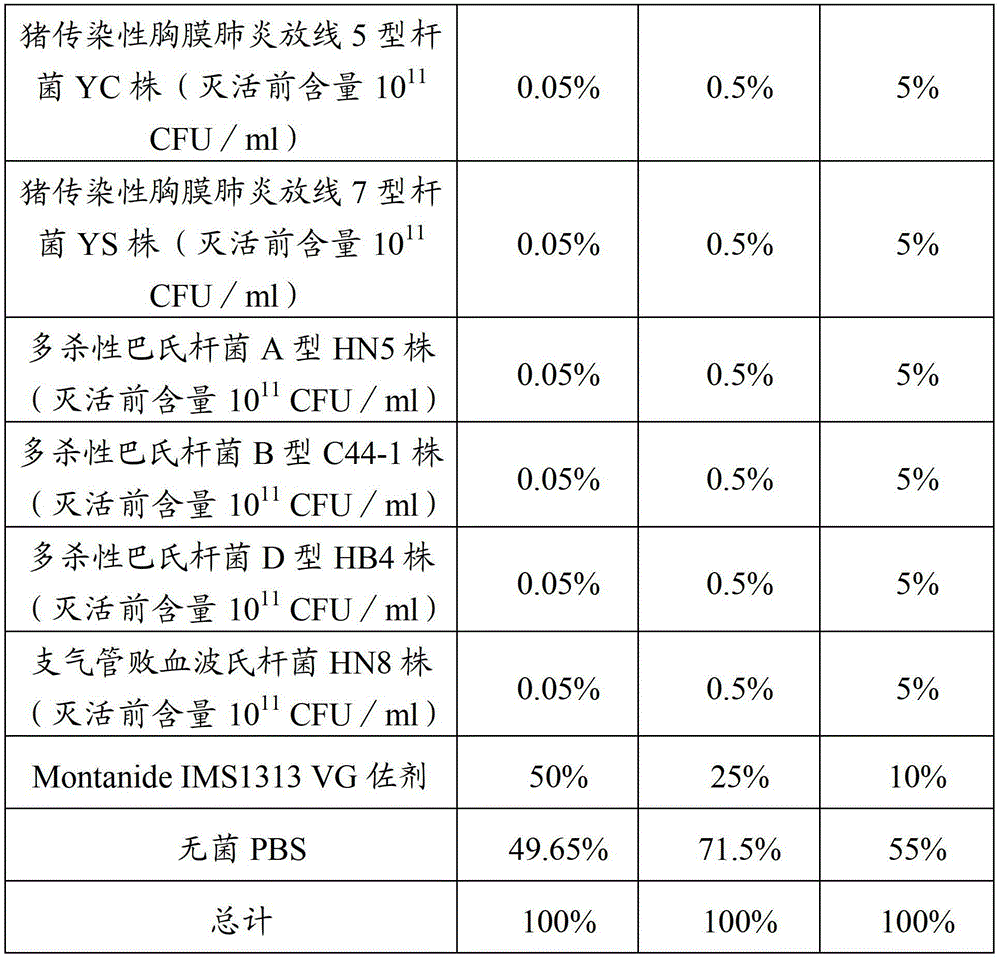
[0038] Implementation 1 to prevent porcine infectious pleuropneumonia, porcine pasteurellosis and porcine atrophic rhinitis inactivated vaccine preparation method:
[0039] 1. Bacteria
[0040] Actinobacillus pleuropneumoniae type 1 LC strain, accession number: CCTCC M 2011458.
[0041] Actinobacillus pleuropneumoniae type 5 YC strain, accession number: CCTCC M 2011459.
[0042] Actinobacillus pleuropneumoniae type YS strain, accession number: CCTCC M 2011460.
[0043] Pasteurella multocida type A HN5 strain, deposit number: CCTCC M 2011222.
[0044] Pasteurella multocida D-type HB4 strain, deposit number: CCTCC M 2011221.
[0045] Pasteurella multocida type B is the capsule type B (group) Pasteurella multocida C44-1 strain, which is identified, kept and supplied by the China Veterinary Drug Administration.
[0046] Bordetella bronchiseptica strain HN8, deposit number: CCTCC M 2011223.
[0047] 2. Preparation of vaccines
[0048] 2.1 Preparation of seeds for production ...
Embodiment 2
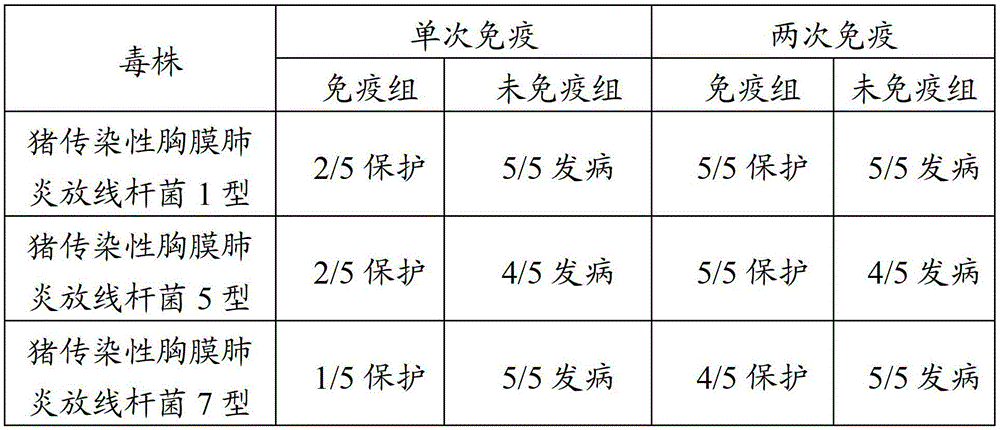
[0100] Example 2 Comparison of the immunization effects of a single vaccine of Actinobacillus pleuropneumoniae, a single vaccine of Pasteurella suis, a single vaccine of Bordetella and the combined vaccine of the present invention
[0101] A total of 210 healthy and susceptible 3-week-old weaned piglets were selected and divided into two groups, 105 in the immunization group and 105 in the control group.
[0102] Among them, 35 piglets in the immunization group were immunized with the inactivated vaccine B of the present invention for porcine infectious pleuropneumonia, pasteurellosis and porcine atrophic rhinitis. The 3-week-old piglets were immunized at an interval of 3 weeks. Each virulent strain was used for challenge, and the method and dose of challenge were shown in 3.3 Efficacy Test in Example 1.
[0103] The other 30 animals in the immunization group were immunized with a single vaccine of Actinobacillus pleuropneumoniae (the antigen content was the number of live bac...
Embodiment 3
[0156] Example 3 Infection model of respiratory disease secondary to atrophic rhinitis
[0157] 6-week-old piglets were selected and divided into 8 groups. All piglets were inoculated with each bacterial venom via the oronasal route according to the protocol in Table 17
[0158] Table 17 Grouping table of infection models for respiratory diseases secondary to atrophic rhinitis
[0159]
[0160]
[0161] Test attack results:
[0162] Group A: The pigs were normal, without any symptoms, and the organs were normal in anatomy.
[0163] Group B: The pigs were normal and did not have any symptoms. The autopsy found that the turbinate bones were slightly atrophied, and there were no lesions in the lungs. No Bordetella was detected in the nasal swab.
[0164] Group C: The piglets had chronic symptoms, with occasional cough symptoms. The autopsy found that there were multiple necrotic foci in the lungs, and cellulose flocs were attached to the pleura and pericardium. The patho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com