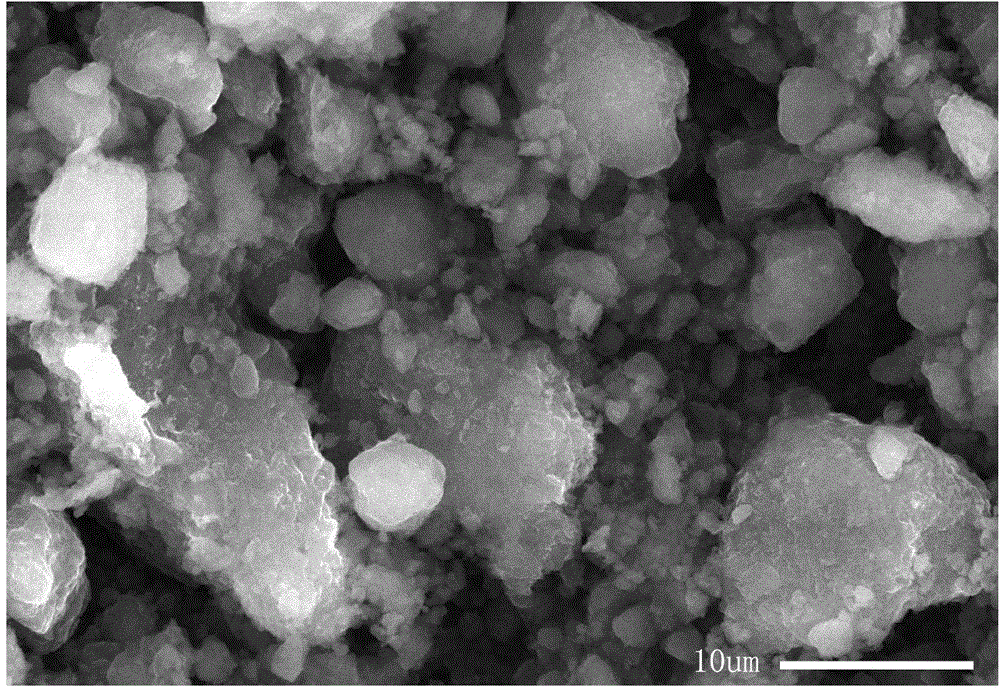
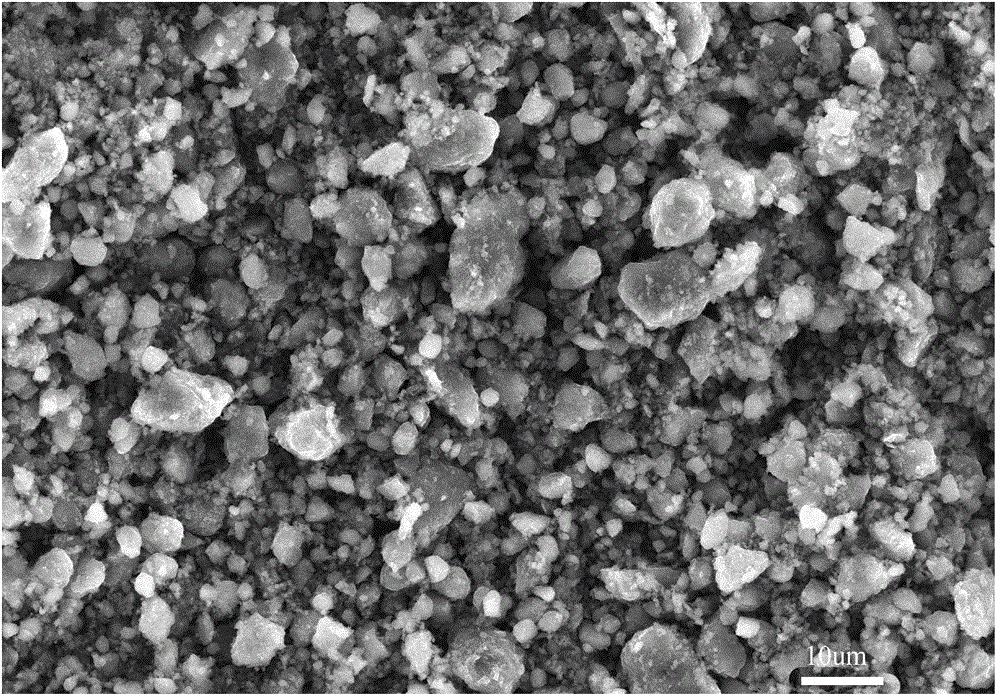
Preparation method of nickel-iron-zirconium compound ferrite
A technology of ferrite and nickel-iron, which is applied in the field of preparation of nickel-iron-zirconium composite ferrite, can solve the problems of lack of overall uniformity of materials, difficulty in mixing to a uniform state, etc., and achieve excellent wave-absorbing ability, uniform mixing, The effect of uniform material composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Take nickel nitrate, ferric nitrate and zirconium tetrachloride with a volume of 20mL to prepare a mixed solution, wherein the concentration of nickel nitrate is 0.2mol / L, the concentration of ferric nitrate is 0.5mol / L, and the concentration of tetrachloride is 0.5mol / L. The molar concentration of zirconium oxide is 0.1mol / L, and at an ultrasonic frequency of 40KHz, add 50mL of 1mol / L sodium hydroxide solution dropwise to the mixed solution, let the precipitate stand for 24h, filter, and wash with hot water for 5 times , and then washed 5 times with ethanol, and the obtained solid precipitate was dried in an oven at 80° C. for 8 hours. After taking it out, it is calcined in a tube furnace. During calcination, the temperature rise program is 8°C / min to 400°C, and it is kept for 1h, and then it is raised to the target temperature of 850°C at 2°C / min, and it is kept for 4h. -5Fe 2 o 3 -ZrO 2 Composite ferrite.
Embodiment 2
[0023] Take nickel nitrate, ferric nitrate and zirconium tetrachloride with a volume of 20mL to prepare a mixed solution, wherein the concentration of nickel nitrate is 0.1mol / L, the concentration of ferric nitrate is 0.8mol / L, and the concentration of tetrachloride is 0.8mol / L. The molar concentration of zirconium oxide is 0.1mol / L, and at an ultrasonic frequency of 40KHz, add 60mL of 1mol / L sodium hydroxide solution dropwise to the mixed solution, let the precipitate stand for 24h, filter, and wash with hot water for 5 times , and then washed 5 times with ethanol, and the obtained solid precipitate was dried in an oven at 80° C. for 8 hours. After taking it out, it is calcined in a tube furnace. During calcination, the temperature rise program is 8°C / min to 400°C, and it is kept for 1 hour, and then it is raised to the target temperature of 850°C at 2°C / min, and it is kept for 4 hours. After cooling down, the solid is taken out to obtain NiO-8Fe 2 o 3 -ZrO 2 .
Embodiment 3
[0025] Take nickel nitrate, iron nitrate and zirconium tetrachloride with a volume of 15mL to prepare a mixed solution, wherein the concentration of nickel nitrate is 0.3mol / L, the concentration of iron nitrate is 0.9mol / L, and the concentration of tetrachloride is 0.9mol / L. The molar concentration of zirconium oxide is 0.1mol / L, and at an ultrasonic frequency of 40KHz, add 60mL of 1mol / L sodium hydroxide solution dropwise to the mixed solution, let the precipitate stand for 24h, filter, and wash with hot water for 5 times , and then washed 5 times with ethanol, and the obtained solid precipitate was dried in an oven at 80° C. for 8 hours. After taking it out, it is calcined in a tube furnace. During calcination, the temperature rise program is 8°C / min to 400°C, and it is kept for 1 hour, and then it is raised to the target temperature of 850°C at 2°C / min, and it is kept for 4 hours. After cooling down, the solid is taken out to obtain 3NiO-4.5Fe 2 o 3 -ZrO 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com