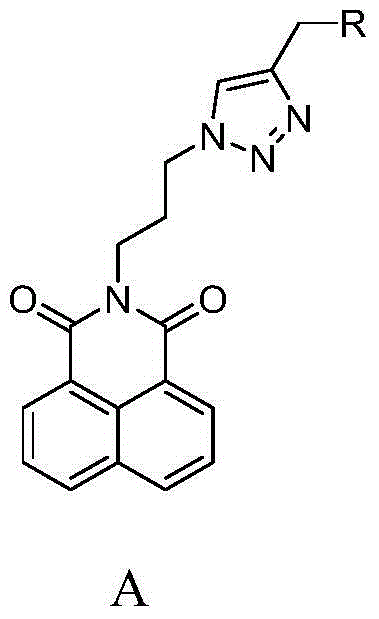
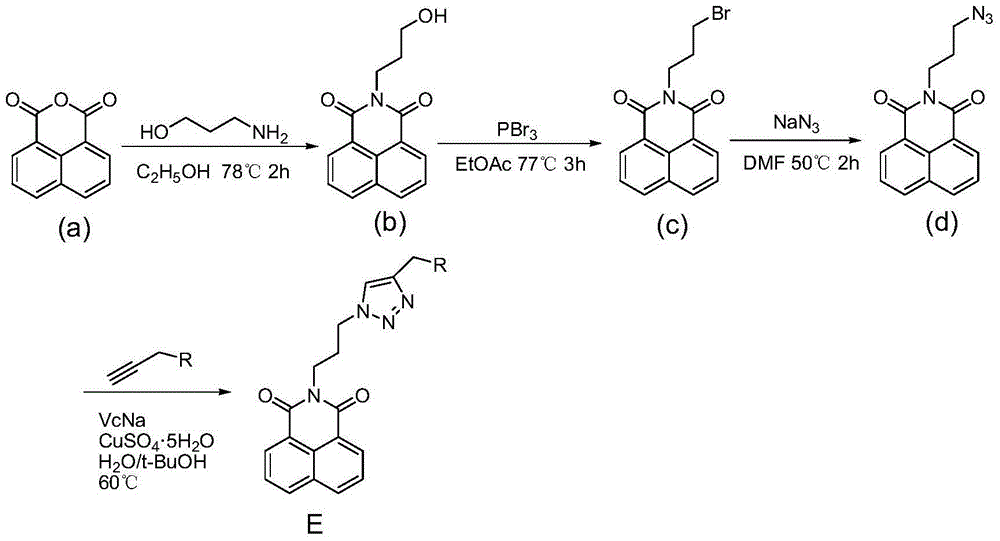
Synthesis and application of naphthalimide derivative containing 1,2,3-triazole on amide side chain
A technology of naphthalimide and derivatives, applied in the field of synthesis of naphthalimide derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Synthesis of N-[3'-(4-morpholinomethyl-[1,2,3]-triazole)-propyl]-1,8-naphthalimide (E1)
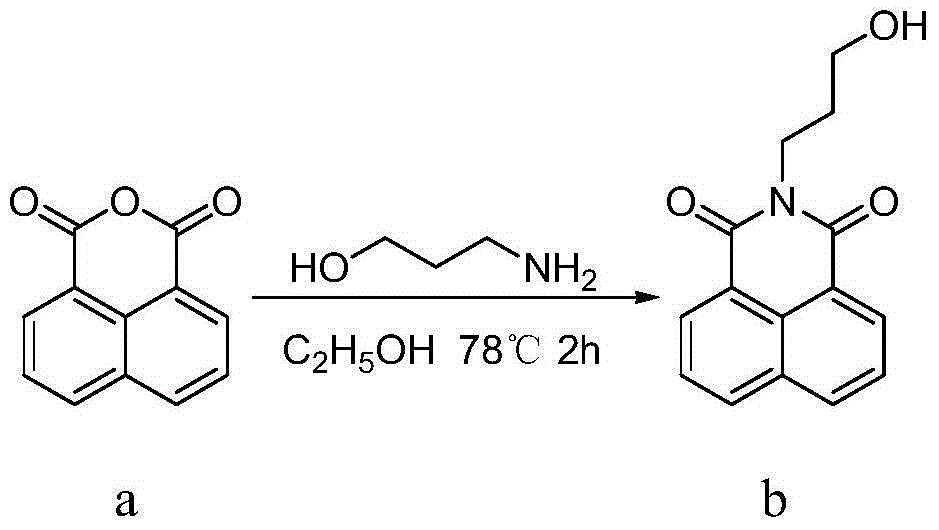
[0021] ①In a 50mL two-necked bottle, add 1.98g naphthalene anhydride and 30mL ethanol, stir evenly, add 0.92mL n-propanolamine, reflux for 2 hours, pour into cold water after standing still, suction filter, wash with water, and dry to obtain the compound b White solid 2.23g, yield 87%.
[0022]
[0023] ②In a 25mL two-necked bottle, add 0.51g of compound b and 5mL of ethyl acetate, stir well at room temperature, and dissolve 380μL of PBr 3 It was slowly dropped into the reaction system, refluxed for 3 hours, and evaporated to obtain a solid. The solid was washed with water and dried to obtain 0.50 g of compound c as a light yellow solid with a yield of 79%.
[0024]
[0025] ③In a 25mL two-necked bottle, add 0.50g of compound c, 8mL of DMF and 0.31g of NaN3, heat to 50°C for 2h, pour into cold water after standing, filter with suction, wash with water, and dry to obtain comp...
Embodiment 2
[0032] Synthesis of N-[3'-(4-thiomorpholinemethyl-[1,2,3]-triazole)-propyl]-1,8-naphthalimide (E2)
[0033] Except that 3-thiomorpholinopropyne was used instead of 3-morpholinopropyne, other synthesis and purification methods were the same as in Example 1 to obtain the target compound E2 as an off-white solid with a yield of 72%.
[0034]
[0035] 1 H NMR (400MHz, CDCl 3 )δ8.61(d, J=6.7Hz, 2H), 8.25(d, J=8.0Hz, 2H), 7.84(s, 1H), 7.79(t, J=7.1Hz, 2H), 4.50(t, J=6.4Hz, 2H), 4.26(t, J=6.7Hz, 2H), 3.83(s, 2H), 3.02–2.85(m, 4H), 2.85–2.68(m, 4H), 2.48–2.34(m ,2H).
[0036] TOF MS(m / z):C 22 h 24 N 5 o 2 S+, Calculated: 422.1651, Found: 422.1644.
Embodiment 3
[0038] Synthesis of N-[3'-(4-piperidinylmethyl-[1,2,3]-triazole)-propyl]-1,8-naphthalimide (E3)
[0039] Except that 3-morpholinopropyne was replaced by 3-piperidylpropyne, other synthesis and purification methods were the same as in Example 1 to obtain the target compound E3 as a gray solid with a yield of 80%.
[0040]
[0041] 1 H NMR (400MHz, CDCl 3 )δ8.57(d, J=7.2Hz, 2H), 8.21(d, J=8.3Hz, 2H), 7.81–7.73(m, 2H), 7.73(s, 1H), 4.45(t, J=7.2 Hz, 2H), 4.25(t, J=6.7Hz, 2H), 3.68(s, 2H), 2.64–2.43(m, 4H), 2.43–2.30(m, 2H), 1.72–1.51(m, 4H) ,1.49–1.34(m,2H).
[0042] TOF MS(m / z):C 23 h 26 N 5 o 2 +, calculated value: 404.2087, measured value: 404.2083.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com