Method for synthetizing minodronic acid intermediate and preparing minodronic acid by virtue of one-pot process
A technology of minodronic acid, synthesis method, applied in the field of chemistry, capable of solving problems such as danger
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
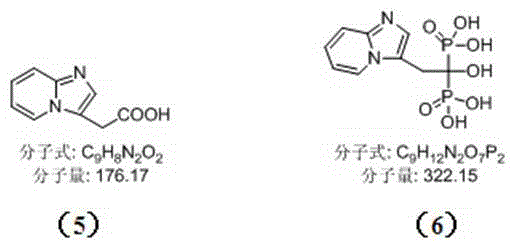
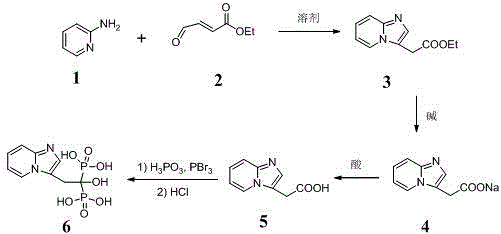
[0010] Example 1: Synthesis of 2-{imidazo[1,2-a]pyridin-3-yl}acetic acid (compound 5)
[0011] Step 1: Add 360 g (3.82 mol) of compound to a 10 L three-necked flask 1 and 3.6 L ethanol, N 2 Protected, stirred in an ice-water bath until the solid was completely dissolved, and controlled the temperature at 0-10 °C; weighed 588 g (4.58 mol) of the compound 2 The reaction system was added dropwise within 1 h, and the temperature of the system rose slightly; after the dropwise addition, the temperature was controlled at 0-10 °C for 2 h, and the reaction progress was monitored by TLC. 2 After the reaction was complete, the reaction was changed to heating and reflux reaction for 6h, and the progress of the reaction was monitored by TLC to obtain the compound 3 .
[0012] Step 2: Change the reaction to an ice-water bath and cool to 0~10 °C; weigh 306 g (7.65 mol) NaOH and add 920 mL ice water to make a solution, and add dropwise to the above reaction system within 30 min after coo...
Embodiment 2
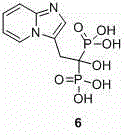
[0016] Example 2: Synthesis of Minodronic Acid (Compound 6)
[0017] Step 1: Prepare a compound with a purity of more than 97% according to the method of Example 1 5 .
[0018] Step 2: Add 1500 mL of chlorobenzene into a 5 L three-necked flask, start adding and stirring, and use nitrogen protection; weigh 60.0 g (0.34 mol) of the compound after 20 min 5 and 84.0 g (1.02 mol) of phosphorous acid were added to the reaction system, and the heating was turned on; when the temperature rose to 90~100 °C, 130 mL (1.37 mol) of phosphorus tribromide was added dropwise to the system within 40 min, and an exhaust gas absorption device was added ; After the dropwise addition was completed, the temperature was controlled at 110-115 °C for reaction, and the progress of the reaction was detected by TLC until the compound 5 After the reaction was complete, the heating was stopped, and the temperature was cooled to room temperature.
[0019] Step 3: A large amount of orange-yellow sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com