A kind of pharmaceutical composition containing zoledronic acid and its preparation
A technology of zoledronic acid and a composition, applied in the field of medicine, can solve the problems of increasing bone mineral density and bone weight coefficient, poor symptom control, increasing blood phosphorus and blood calcium content, etc., so as to reduce toxic side effects and improve drug use. Compliance and therapeutic effect, effect of stable content of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
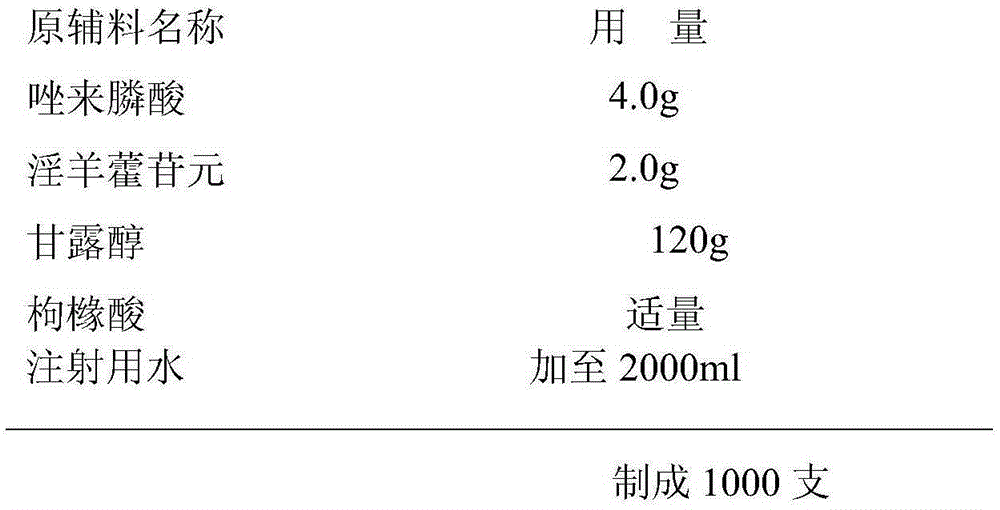
[0022] The freeze-dried powder injection of embodiment 1 pharmaceutical composition of the present invention
[0023] Prescription composition:
[0024]
[0025] Preparation process: Weigh zoledronic acid, icariin and excipients respectively according to the prescription amount, add the excipients into an appropriate amount of water for injection, stir to dissolve and adsorb with 0.1% activated carbon, add zoledronic acid, stir to dissolve and dissolve with citric acid Adjust the pH value to 6.0-7.0, sterilize and filter with 0.45 μm and 0.22 μm filters after constant volume, fill, half-tamp, and freeze-dry to obtain the freeze-dried powder injection of the composition of the present invention.
Embodiment 2
[0026] The freeze-dried powder injection of embodiment 2 pharmaceutical composition of the present invention
[0027] Prescription composition:
[0028]
[0029] The preparation method is the same as Example 1 of the present invention except that the prescription is different.
Embodiment 3
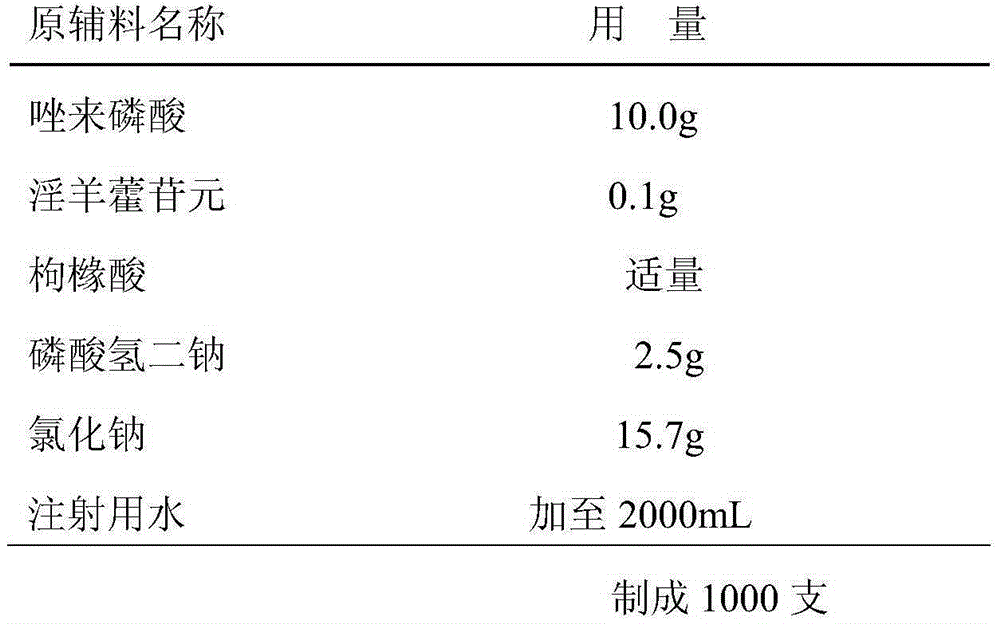
[0030] The freeze-dried powder injection of embodiment 3 pharmaceutical composition of the present invention
[0031] Prescription composition:
[0032]
[0033]
[0034] The preparation method is the same as Example 1 of the present invention except that the prescription is different.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com