Submicron nucleocapsid microsphere material capable of releasing active factors on time and preparation method of submicron nucleocapsid microsphere material
A technology for releasing active, core-shell microspheres, applied in medical science, prosthesis, etc., can solve the problems such as the inability to obtain the temporal synergy of osteogenesis and angiogenesis, the size effect of core-shell microspheres is difficult to meet the clinical transformation application, etc. Strong controllability, beneficial to clinical transformation and application, simple preparation and processing methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Weigh 0.4g poly-D,L-lactic acid (PDLLA), add 10mL trifluoroethanol solution to swell at room temperature for 1h, and then stir at 37°C for 24h to form a uniform shell solution of 2% w / v. Weigh 0.2 g of PLGA, add 10 mL of trifluoroethanol solution to swell at room temperature for 1 h, and then stir at 37° C. for 24 h to form a 4% w / v uniform core layer solution.
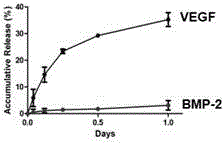
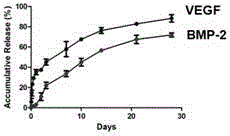
[0027] (2) Dissolve VEGF and BMP‐2 growth factors in ultrapure water respectively, add VEGF solution to the shell layer solution at a ratio of 10nm / ml, and add BMP‐2 solution to the core layer solution at a ratio of 100nm / ml , respectively stirred for 10 min to form a uniform and stable core and shell solution.
[0028] (3) Inject the core and shell solutions obtained in step (2) into 20mL medical syringes respectively, and place them in a coaxial electrostatic spray device to spray microspheres with a spray voltage of 15kV, a total flow rate of 0.8ml / h, and a core-shell flow rate ratio of 1. : 3, the rece...
Embodiment 2
[0032] (1) Prepare 1% w / v PDLLA trifluoroethanol core layer solution, swell at room temperature for 1 hour, and stir at 37°C for 24 hours; prepare 3% w / v PLGA trifluoroethanol core layer solution, swell at room temperature for 1 hour, and stir at 37°C 24h.
[0033] (2) Dissolve VEGF and BMP‐2 growth factors in ultrapure water respectively, add VEGF solution to the nuclear layer solution at a ratio of 10nm / ml, and add BMP‐2 solution to the nuclear layer solution at a ratio of 100nm / ml , respectively stirred for 10 min to form a uniform and stable core and core layer solution.
[0034] (3) Inject the core and layer solutions obtained in step (2) into 20mL medical syringes respectively, place them in a coaxial electrostatic spraying device to spray microspheres, the spraying voltage is 15kV, the total flow rate is 0.8ml / h, and the core-shell flow rate ratio is 1: 4. The receiving board distance is 20cm.
[0035] (4) Dry the product obtained in step (3) in a vacuum freeze dryer ...
Embodiment 3
[0038] (1) Prepare 1% w / v PDLLA trifluoroethanol core layer solution, swell at room temperature for 1 hour, and stir at 37°C for 24 hours; prepare 3% w / v PLGA trifluoroethanol core layer solution, swell at room temperature for 1 hour, and stir at 37°C 24h.
[0039] (2) Dissolve BMP-2 and VEGF growth factors in ultrapure water respectively, add BMP-2 solution to the nuclear layer solution at a ratio of 100nm / ml, and add VEGF solution to the nuclear layer solution at a ratio of 10nm / ml , respectively stirred for 10 min to form a uniform and stable core and core layer solution.
[0040] (3) Inject the core and layer solutions obtained in step (2) into 20mL medical syringes respectively, place them in a coaxial electrostatic spraying device to spray microspheres, the spraying voltage is 15kV, the total flow rate is 0.8ml / h, and the core-shell flow rate ratio is 1: 2.
[0041] (4) Dry the product obtained in step (3) in a vacuum freeze dryer for 24 hours, and store it sealed at -...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com