Method for preparing A type atazanavir sulfate
A technology of atazanavir sulfate and concentrated sulfuric acid, applied in directions such as organic chemistry, can solve problems such as cumbersome operation, unfavorable for industrialized production, etc., and achieve the effects of low cost and short preparation period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Atazanavir free base (150g, 212.8mmol) is added in the mixed solvent that is formed by 2250mL ethyl acetate and 150mL DMSO, stirs and heats up under argon atmosphere, after the system dissolves clear, add 1.5g gac decolorization (system temperature is at 50~60℃), filter to obtain a clear solution; control the temperature at 35±5℃, add concentrated sulfuric acid (11.82mL, 212.8mmol) dropwise to the prepared clear solution; after dropping, keep stirring for 1~2 hours; Slowly (about 30-60 minutes) cool down to 0-15°C, filter, wash the filter cake with ethyl acetate 2-3 times, and vacuum-dry at 40-60°C for 5-8 hours to obtain 158g of atazanavir sulfate , the molar yield was 92.5%, the HPLC purity was >99.5%, and the maximum simplex was <0.1%.
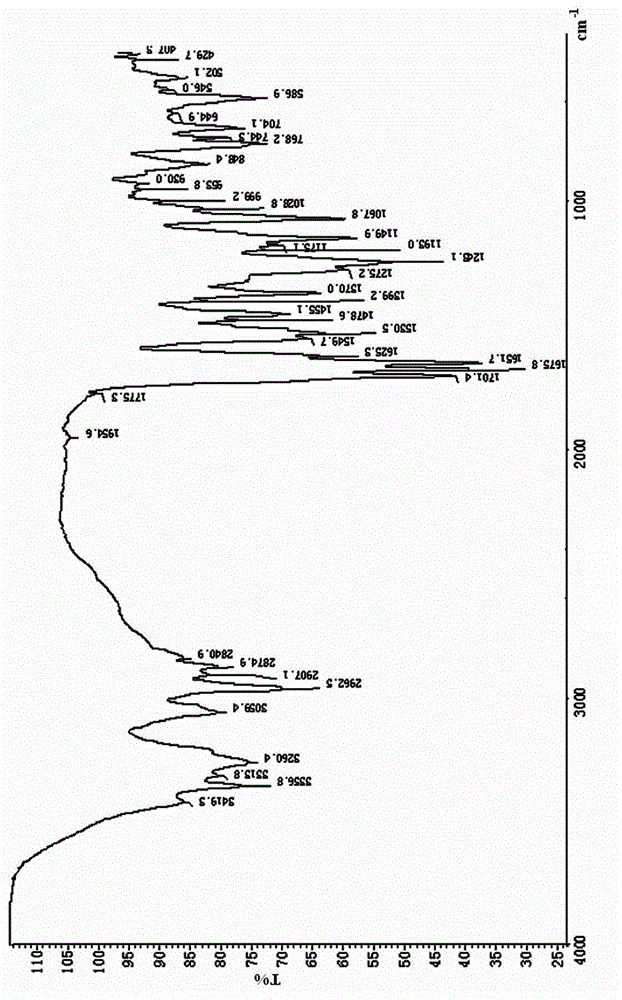
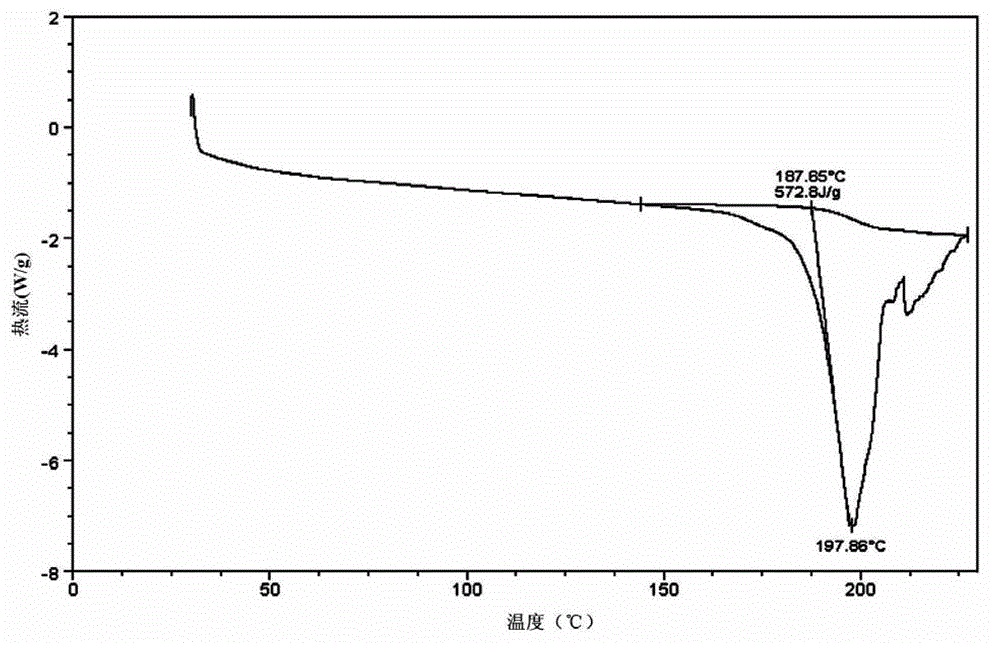
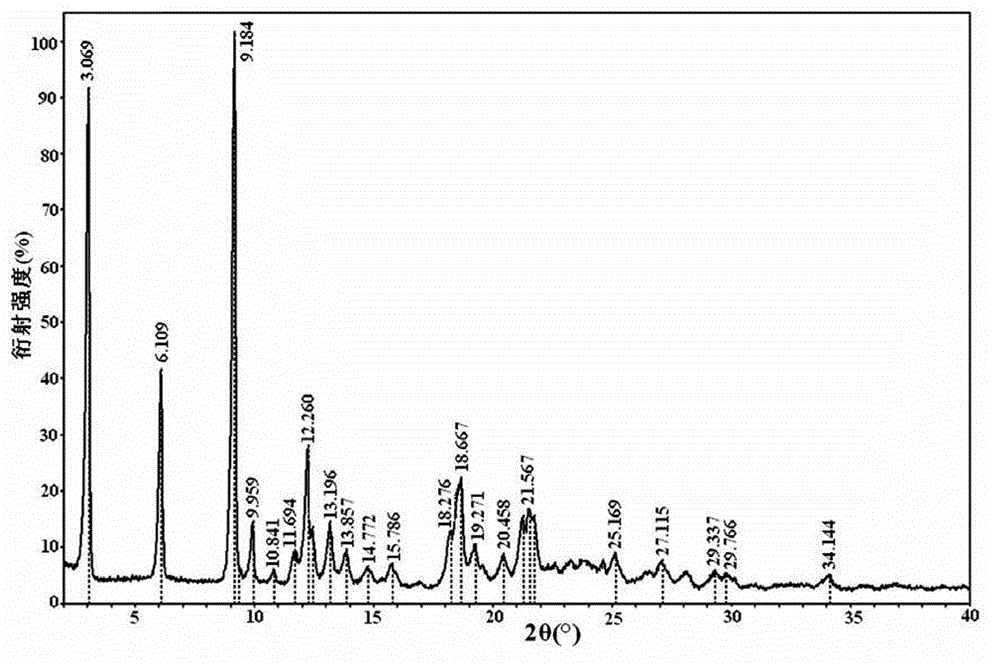
[0030] The FTIR collection of illustrative plates of the atazanavir sulfate that the present embodiment obtains is as figure 1 As shown, the DSC spectrum is as figure 2 As shown, the XRPD pattern is as image 3 Shown; Because the...
Embodiment 2
[0032] Atazanavir free base (10g, 14.19mmol) is added in the mixed solvent that is formed by 120mL ethyl acetate and 10mL DMSO, stirs and heats up under argon atmosphere, after the system dissolves clear, add 0.1g active carbon decolorization (system temperature is at 50~60℃), filter to obtain a clear solution; control the temperature at 35±5℃, add concentrated sulfuric acid (0.8mL, 14.4mmol) dropwise to the prepared clear solution; after dropping, keep stirring for 1~2 hours; Slowly (about 30-60 minutes) cool down to 0-15°C, filter, wash the filter cake with ethyl acetate 2-3 times, and vacuum-dry at 40-60°C for 5-8 hours to obtain atazanavir sulfate 10.6 g, the molar yield is 93.0%, the HPLC purity is >99.5%, and the maximum simplex is <0.1%.
[0033] The atazanavir sulfate that the present embodiment obtains also has figure 1 The FTIR spectrum shown, figure 2 The DSC spectrum shown and image 3 The XRPD pattern shown is for atazanavir sulfate Form A.
Embodiment 3
[0035] Atazanavir free base (10g, 14.19mmol) is added in the mixed solvent that is formed by 120mL isopropyl acetate and 10mL DMSO, stirs and heats up under argon atmosphere, after the system dissolves clear, add 0.1g activated carbon decolorization (system temperature (50-60°C), filter to obtain a clear solution; control the temperature at 35±5°C, add concentrated sulfuric acid (0.8mL, 14.4mmol) dropwise to the prepared clear solution; after dropping, keep stirring for 1-2 hours Slowly (about 30-60 minutes) cool down to 0-15°C, filter, wash the filter cake with isopropyl acetate for 2-3 times, and vacuum-dry at 40-60°C for 5-8 hours to obtain atazanavir sulfate Salt 10.3g, molar yield 90.4%, HPLC purity > 99.5%, maximum simplex < 0.1%.
[0036] The atazanavir sulfate that the present embodiment obtains also has figure 1 The FTIR spectrum shown, figure 2 The DSC spectrum shown and image 3 The XRPD pattern shown is for atazanavir sulfate Form A.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com