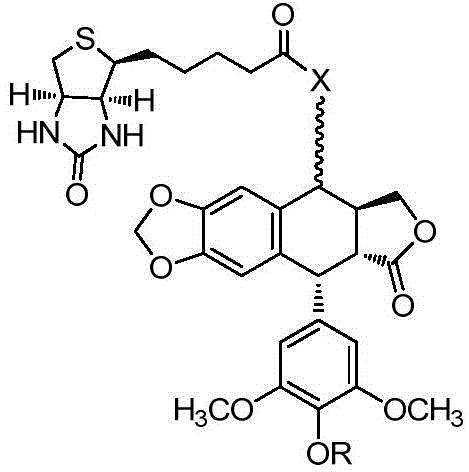
Biotin-podophyllotoxin esterified derivative and pharmaceutical composition thereof, as well as preparation methods and applications of derivative and pharmaceutical composition
A technology of podophyllotoxin and biotin, which is applied in drug combination, pharmaceutical formulation, organic chemistry, etc., can solve the problems of no anti-tumor activity of such compounds, no biotin-podophyllotoxin ester derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
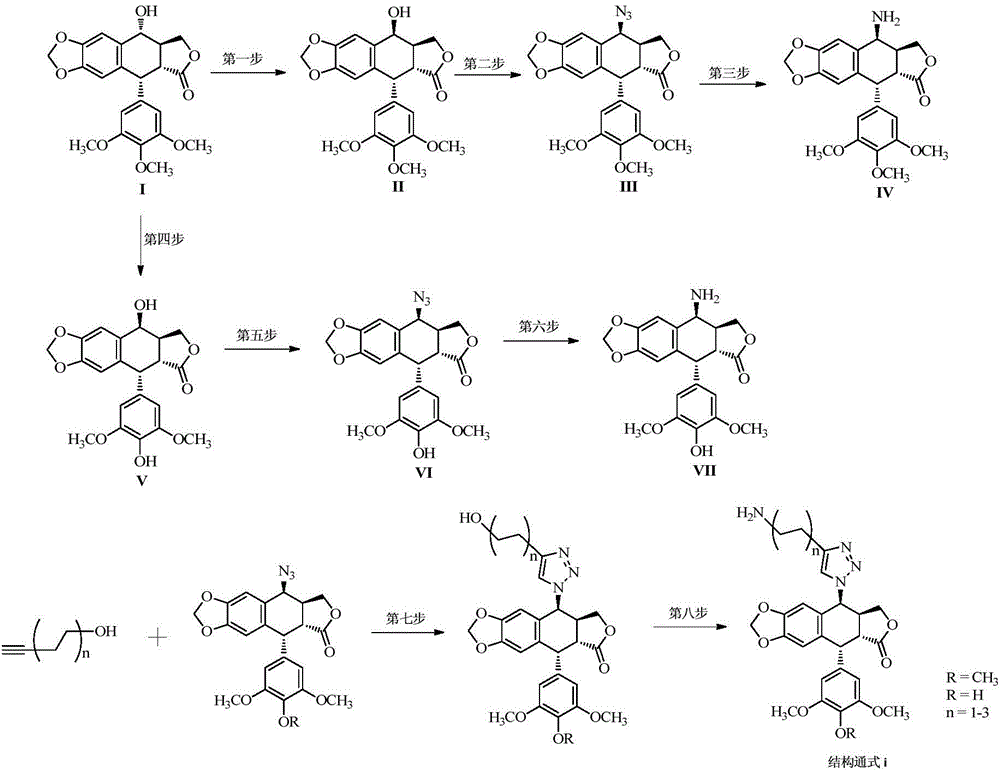
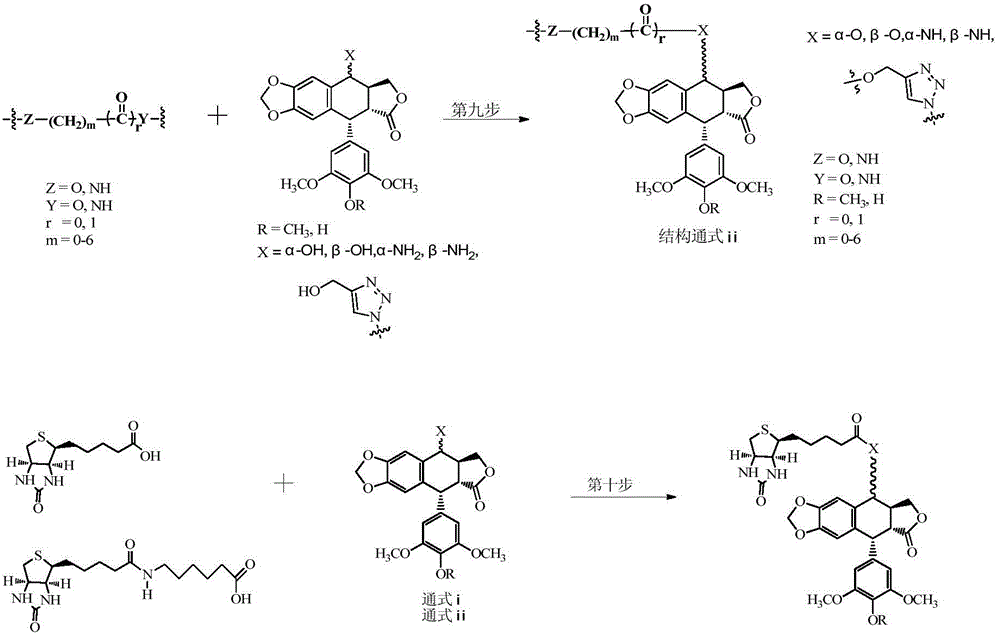
[0037] Synthetic general formula of the present invention such as figure 2 shown.
Embodiment 2
[0039] The preparation of compound 4β-amino-(biotin)-4-deoxy-4'-demethylpodophyllotoxin: (by figure 2 The fourth step, the fifth step, the sixth step, and the tenth step in the middle)
[0040] the fourth step: Preparation of 4'-O-desmethylpodophyllotoxin (V):
[0041] 2.1 g of podophyllotoxin (5.0 mmol) was dissolved in dry dichloromethane solution, sodium iodide (2.3 g, 15.0 mmol) was added to the above solution, and stirred for 5 minutes. The reaction liquid was cooled to 0° C. in an ice-water bath, and 1.4 g of methanesulfonic acid (15.0 mmol) was slowly added dropwise to the above reaction liquid to continue stirring, and then the reaction was continued at room temperature for 5 hours. Nitrogen protection was used to remove the HI gas generated during the reaction. Then, the solvent in the above reaction was removed under reduced pressure, and the next step was carried out without any treatment. Dissolve the above crude product in acetone-water (25mL-25mL) mixed sol...
Embodiment 16
[0131] In vitro inhibitory effects of biotin-podophyllotoxin ester derivatives on different tumor cells:
[0132] 1. Experimental materials
[0133]Cell lines: human leukemia cell line HL-60, human liver cancer cell line SMMC-7721, human lung cancer cell line A-549, human breast cancer cell line MCF-7, and human colon cancer cell line SW480.
[0134] Detection principle: MTT method to detect cell viability
[0135] 2. Test method
[0136] 1). Inoculate cells: use culture medium (DMEM or RMPI1640) containing 10% fetal bovine serum to prepare a single cell suspension, inoculate 5000–10000 cells per well into a 96-well plate with a volume of 100 μl per well, adherent cells Inoculate the culture 12 hours in advance.
[0137] 2). Add the compound solution to be tested (preliminary screening at a fixed concentration of 40 μM, at which concentration the compound that inhibits tumor cell growth near 50% is set to enter the gradient rescreening at 5 concentrations), the final volume...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com