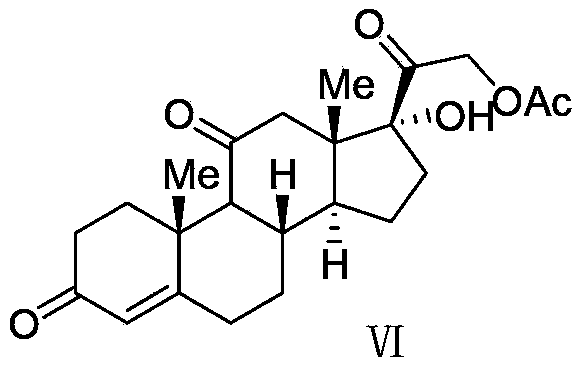
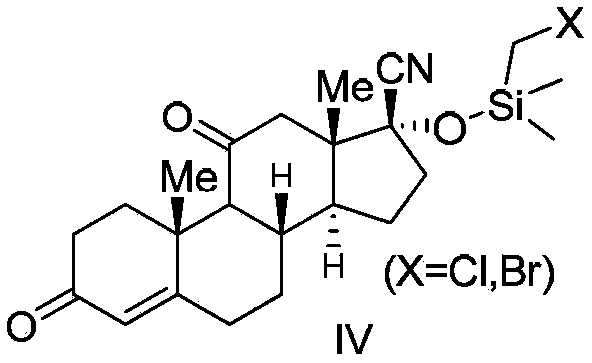
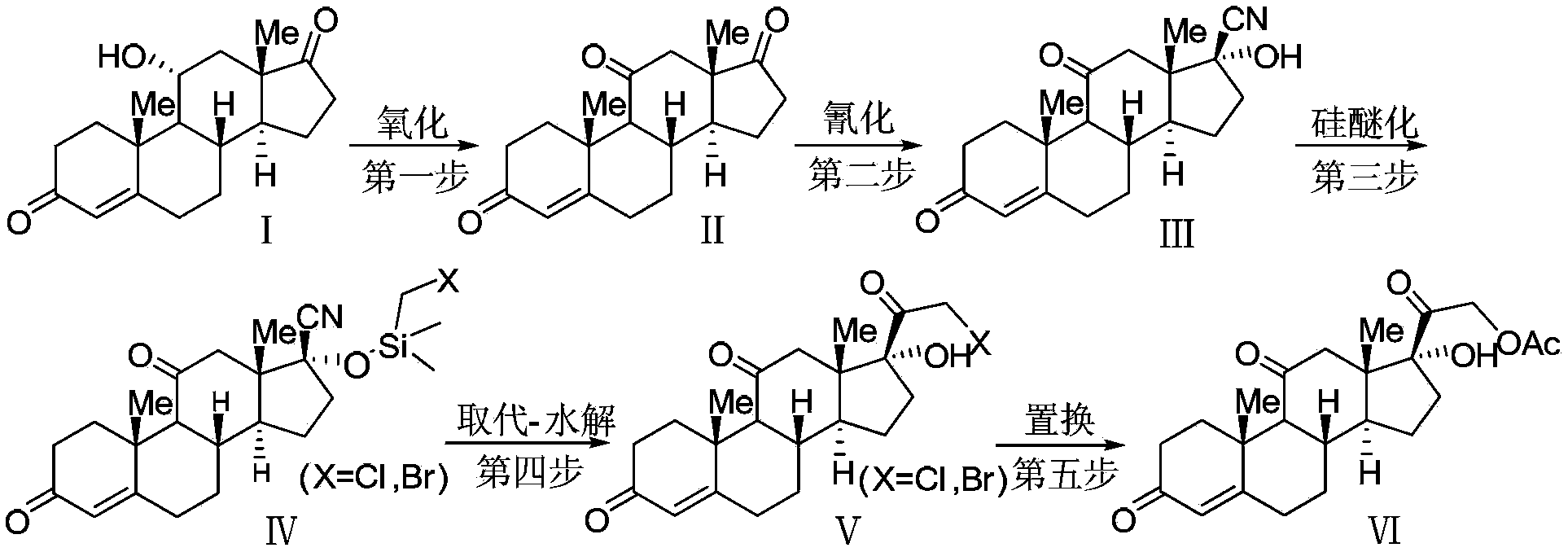
Cortisone acetate intermediate, preparation method and use thereof in preparation of cortisone acetate
A technology of acetate and strong acid, applied in the field of medicine and chemical industry, can solve the problems of low yield, long route, high cost, etc., and achieve the effect of high yield, strong operability, simple and stable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of 4-ene-pregna-3,11,17-trione (compound of formula II)
[0040] Add 4-ene-pregna-11α-hydroxyl-3,17-dione (compound of formula I) (100 g) (obtained through commercial purchase) and 2-butanone (800 ml) in a 2L reaction flask, and cool to 0 At about -10°C, add Jones reagent (150ml) dropwise (calculated as chromium trioxide, 1.1 equivalents), and the addition is completed in about 0.5 hours. After the raw materials are completely consumed, stop the reaction, stir at room temperature, add isopropanol (3ml) to destroy the oxidant, stir for 0.5h, add water (1L) to stir, let stand to separate the layers, separate the organic phase, and use ethyl acetate (200ml ) extraction, combined the organic phase, and added the organic phase to the rotary evaporator for concentration. When about half of the solvent remained, add water (about 500ml) to continue the concentration until the solvent was completely evaporated, and the product was precipitated in water as a white pow...
Embodiment 2
[0042] Preparation of 4-ene-pregna-3,11,17-trione (compound of formula II)
[0043] Add 4-ene-pregna-11α-hydroxy-3,17-dione (compound of formula Ⅰ) (50g), acetone (100ml) into a 2L reaction flask, cool down to about 0-10°C in an ice bath, and add Jones reagent dropwise (75ml) (calculated as chromium trioxide, 1.1 equivalent), about 0.5 hours to complete the addition, after the addition, keep warm overnight. When there is almost no remaining raw material, stop the reaction, stir at room temperature, add isopropanol (1ml) to destroy the oxidant, and concentrate directly after stirring for 0.5h. When about half of the solvent remains, add water (about 500ml) and continue to concentrate until the solvent is completely evaporated. The product was precipitated in water as a light brown powdery solid. Dry at 50°C to obtain 48.1 g of the product with a purity of about 98% and a yield of 97%.
Embodiment 3
[0045] Preparation of 4-ene-pregna-3,11,17-trione (compound of formula II)
[0046] Add 4-ene-pregna-11α-hydroxyl-3,17-dione (compound of formula I) (200g), dichloromethane (1000ml), water (400ml) into the 3L reaction flask, cool to 0-10 At about ℃, Jones reagent (290ml) (calculated as chromium trioxide, 1.1 equivalents) was added dropwise. The addition was completed in about 50 minutes, and after the addition was completed, the reaction was incubated overnight. After the reaction of the raw materials is complete, add isopropanol (6ml) to destroy the oxidant, stir for 1 hour and then separate the layers, separate the organic phase, extract the aqueous phase with dichloromethane (100ml x 1), combine the organic phases, and wash with water (200ml x 1). Wash to remove inorganic matter, add water to the organic phase at a volume ratio of 1:1, and then concentrate under reduced pressure. The product precipitates in the water phase as a white solid. After filtration, it is dried at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com