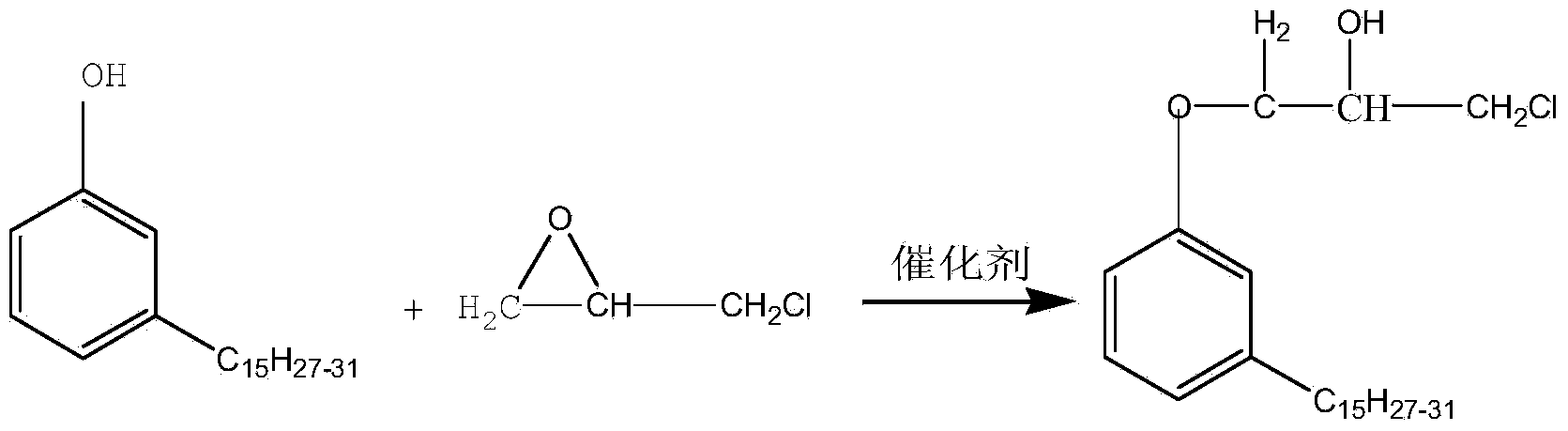
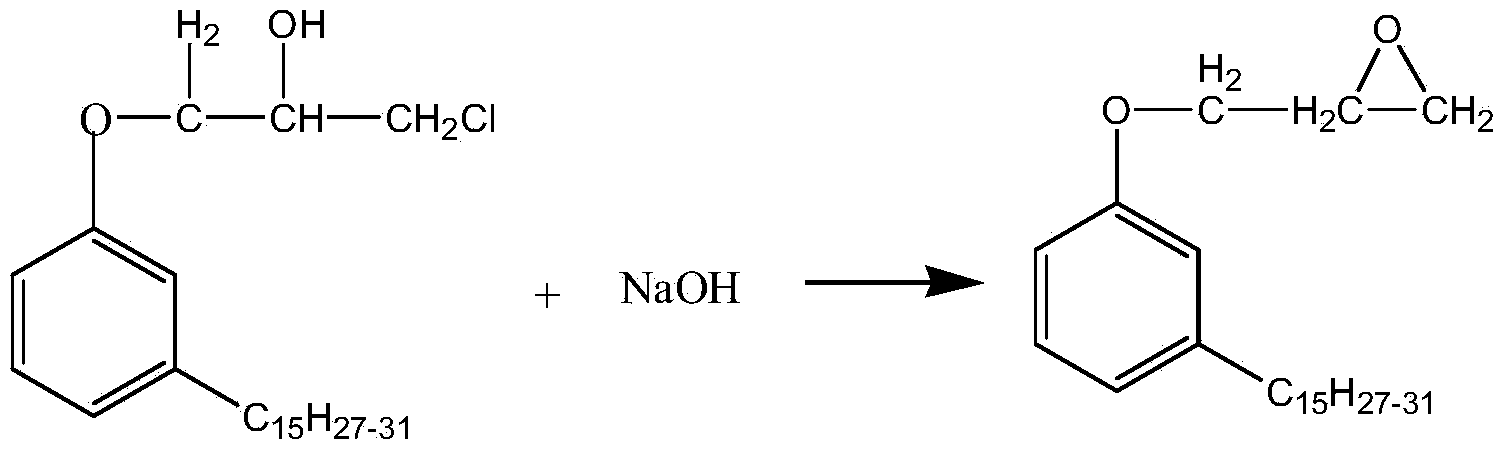Preparation method of plant monophenol glycidyl ether
A technology of glycidyl ether and monophenol, which is applied in the field of plant monophenol to prepare epoxy resin, can solve the problems of poor quality and high epoxy equivalent of plant phenol glycidyl ether
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Pass nitrogen into a 1L four-neck flask equipped with a stirring, thermometer and condensing reflux device to replace the air, add 200g of plant monophenol, 100g of epichlorohydrin, and 0.2g of tetraethylammonium bromide, stir and heat up to 50°C, and open Cycloaddition etherification reaction 1h. Keep the temperature constant, add 40 g of sodium hydroxide solution with a mass concentration of 30% dropwise with a constant pressure funnel, the dropwise addition time is 1 h, and keep for 0.5 h after the dropwise addition to complete the initial ring-closing epoxidation dechlorination reaction. Distill under reduced pressure to recover water and excess epichlorohydrin. The temperature was raised to 60° C., and 20 g of sodium hydroxide solution with a mass concentration of 10% was added at one time to carry out a second ring-closing epoxidative dechlorination reaction for 5 hours. Centrifugal filtration to obtain crude plant monophenol glycidyl ether; washing, liquid separ...
Embodiment 2
[0028] Pass nitrogen into a 1L four-neck flask equipped with a stirring, thermometer and condensing reflux device to replace the air, add 150 g of plant monophenols, 150 g of epichlorohydrin, 0.2 g of tetramethylammonium bromide, and 0.2 g of tetraethylammonium bromide g, stirring and raising the temperature to 75°C, the ring-opening addition etherification reaction was carried out for 2 hours. Keeping the temperature constant, add 45 g of sodium hydroxide solution with a mass concentration of 50% dropwise through a constant pressure funnel for 2.5 hours. Distill under reduced pressure to recover water and excess epichlorohydrin. The temperature was raised to 70° C., and 30 g of sodium hydroxide solution with a mass concentration of 20% was added at one time to carry out a second ring-closing epoxidative dechlorination reaction for 3 hours. Centrifugal filtration to obtain crude plant monophenol glycidyl ether; washing, liquid separation, distillation, and repeated filtration...
Embodiment 3
[0030] Pass nitrogen into a 1L four-neck flask equipped with a stirring, thermometer and condensing reflux device to replace the air, add 100g of plant monophenol, 0.5g of benzyltriethylammonium bromide, stir and raise the temperature to 80°C, and etherify by ring-opening addition Reaction 0.5h. Keeping the temperature constant, 50 g of sodium hydroxide solution with a mass concentration of 60% was added dropwise with a constant pressure funnel for 4 hours, and kept for 3 hours after the dropwise addition to complete the initial ring-closing epoxidative dechlorination reaction. Distill under reduced pressure to recover water and excess epichlorohydrin. The temperature was raised to 90° C., and 40 g of sodium hydroxide solution with a mass concentration of 30% was added at one time to carry out a second ring-closing epoxidative dechlorination reaction for 1 hour. Centrifugal filtration to obtain crude plant monophenol glycidyl ether; washing, liquid separation, distillation, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| epoxy equivalent | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com