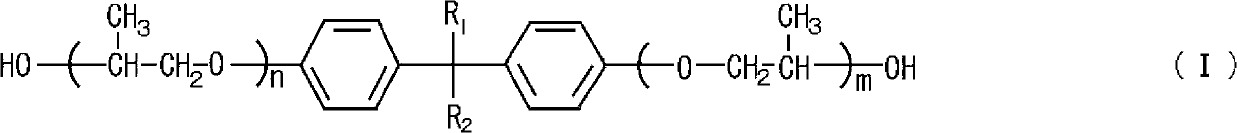
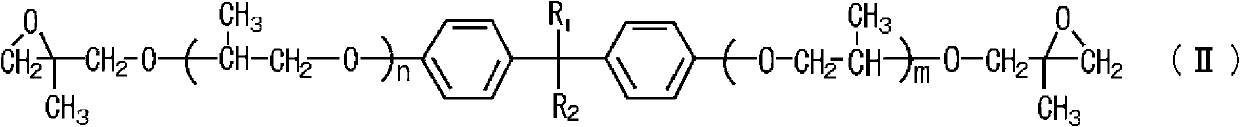
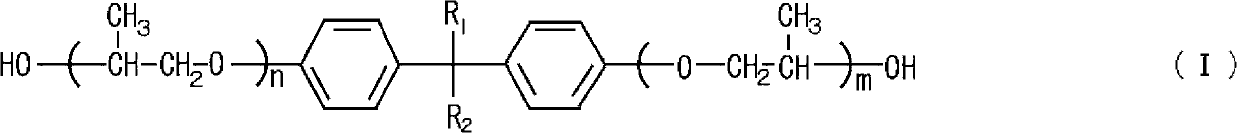
Process for producing polyglycidyl ether
A technology of polyglycidyl ether and a manufacturing method, applied in the directions of organic chemistry, organic chemistry, etc., can solve the problems of no research, difficulty in obtaining the target substance, and high chlorine content, and achieves a small epoxy equivalent and a chlorine-containing The effect of low rate and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 178.9 g (0.5 mol) of a propylene oxide adduct of bisphenol A having a hydroxyl value of 314 (BPX-11 manufactured by ADEKA Co., Ltd.), 462.5 g (5.0 mol) of β-methyl epichlorohydrin, 1.5 g of tetramethylammonium chloride (0.84 parts by mass relative to 100 parts by mass of polyol) was placed in a glass flask equipped with a thermometer, a stirrer, and a cooling tube, and the inside of the flask was heated to 60° C. to maintain the internal pressure. At 11.3kPa and reflux.
[0037] While maintaining the temperature and pressure of the reaction system, 108.3 g of 48.5% by mass sodium hydroxide aqueous solution (1.3 moles of sodium hydroxide) was added dropwise for 90 minutes, aged at 60°C for 150 minutes, and the product produced in the reaction system was filtered. of salt. After distilling off excess β-methylepichlorohydrin in the filtrate at 120°C under reduced pressure, add toluene and wash thoroughly with water, then distill off toluene under reduced pressure and filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com