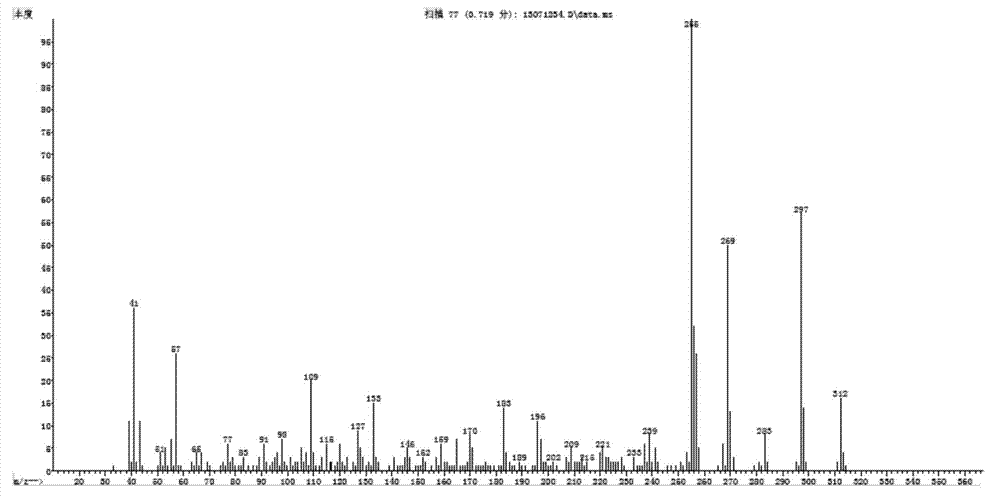
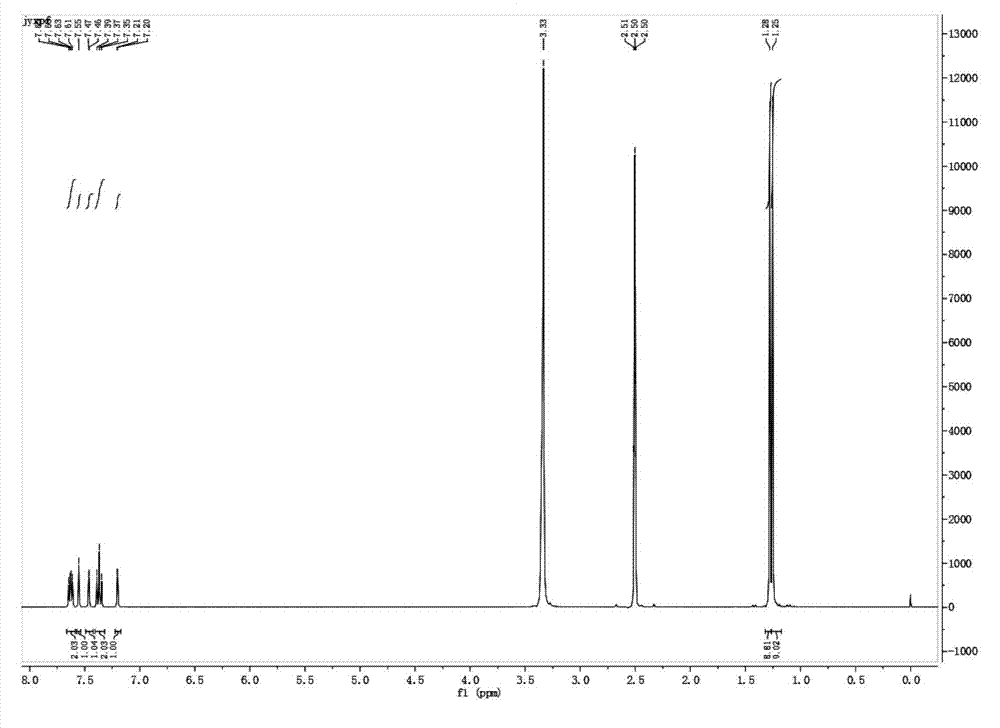
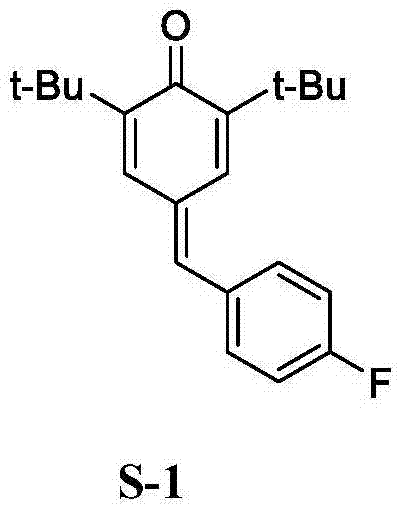
2,6-di-tert-butyl-4-(-4-fluorobenzylidene)-2,5-cyclohexadiene-1-one and preparation method thereof
A technology of fluorobenzylidene and di-tert-butylphenol, which is applied in the field of organic compounds and their synthesis, can solve the problems of high operation and equipment requirements, large environmental pollution, high price, etc., so as to improve the conversion rate of raw materials and reduce the The effect of reaction yield and accelerated formation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1, a preparation of 2,6-di-tert-butyl-4-(-4-fluorobenzylidene)-2,5-cyclohexadien-1-one (QM-ph-p-F) method, follow the steps below:
[0049] A. Preparation of Mannich bases
[0050] Add 41.2g (0.2mol) of 2,6-di-tert-butylphenol and 34.8g (0.28mol) of p-fluorobenzaldehyde into a 250mL three-necked flask with a condensing water separator and a stirrer, stir and heat to a small amount of reflux (about 145°C), after reflux for 20 minutes, keep warm and slowly add 20mL (0.2mol) of piperidine dropwise, and drop it in 3 hours. During the reaction process, the generated water will be evaporated. Monitored by GC.
[0051] B. Mannich base removal of secondary amines
[0052] Transfer the above reaction solution into a 250mL single-necked flask, carry out vacuum distillation under the pressure of 150mmHg, heat the flask with an oil bath, set the oil temperature to 120°C, and continue the distillation for about 3 hours. After the kettle liquid is cooled, a reddish-brown s...
Embodiment 2
[0056] Example 2, a preparation of 2,6-di-tert-butyl-4-(-4-fluorobenzylidene)-2,5-cyclohexadien-1-one (QM-ph-p-F) method, follow the steps below:
[0057] A. Preparation of Mannich bases
[0058] Add 41.2g (0.2mol) of 2,6-di-tert-butylphenol and 34.8g (0.28mol) of p-fluorobenzaldehyde into a 250mL three-necked flask with a condensing water separator and a stirrer, stir and heat to a small amount of reflux (about 145°C), after reflux for 20 minutes, keep warm and slowly drop into about 60.8mL of 33wt% dimethylamine aqueous solution (containing dimethylamine 0.4mol), drop it in 3 hours, steam the water generated during the reaction, continue to keep warm after dropping The reaction was refluxed for 3 hours, the reaction was stopped, and GC was used to monitor during the reaction.
[0059] B. Mannich base removal of secondary amines
[0060] Transfer the above reaction solution into a 250mL single-necked flask, carry out vacuum distillation under the pressure of 150mmHg, heat ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com