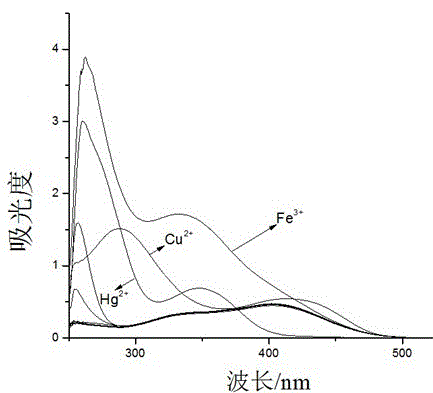
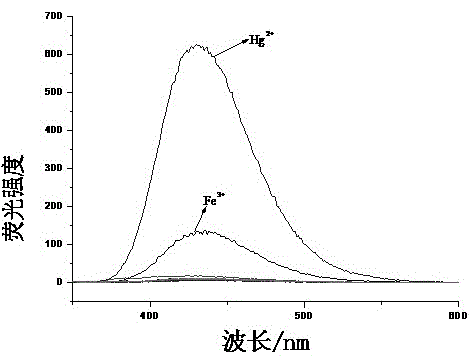
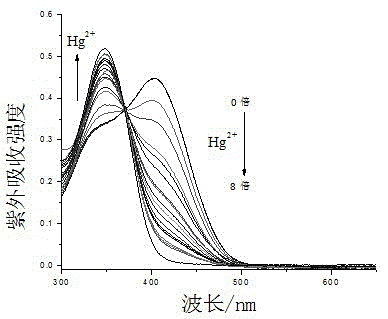
Colorimetric-fluorescent dual-channel recognition of mercury ion acceptor compound and its preparation and application
A compound, mercury ion technology, applied in the field of cation detection, can solve the problems of inability to meet the needs of cheap and fast on-site detection, low sensitivity, and failure to reach the limit value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Synthesis of receptor compounds
[0040] (1) Synthesis of intermediate 5-(nitrophenyl)furan-2-carbaldehyde: the method is as follows: mix 4.143 g of p-nitroaniline, 8 mL of concentrated hydrochloric acid and 16 mL of water and slowly heat to reflux until the nitroaniline is completely dissolved . After the dissolution is complete, place the round-bottomed flask in an ice bath while accelerating stirring to precipitate tiny crystals. Weigh 6 grams of NaNO 2 Dissolve it in 9mL water, slowly add it dropwise into the aniline solution with a constant pressure funnel, stir and react for 20 minutes after dropping, filter, transfer the filtrate to a 250mL round-bottomed flask, add 2.9g of furfuraldehyde, 20mL of acetone and 40 Milliliter of water, stir under ice bath, dissolve 0.8 g of copper chloride in 3 mL of water, slowly add it dropwise, remove the ice bath after dripping, continue to react at room temperature for 8 hours, a brick red precipitate is formed, filter, us...
Embodiment 2
[0047] 1. Synthesis of receptor compounds
[0048] (1) Synthesis of intermediate 5-(nitrophenyl)furan-2-carbaldehyde: same as in Example 1.
[0049] (2) Synthesis of acceptor compound: Add about 1.1g (5mmol) of 5-(nitrophenyl)furan-2-carbaldehyde and about 0.8g (5.5mmol) of α-naphthylamine into a 50mL round bottom flask, Dissolve in 30mL of hot EtOH, and add 1mL of AcOH as a catalyst, then reflux at 85°C for 4h, cool to room temperature, filter the brown precipitate with suction, wash the precipitate with hot EtOH three times, and finally use DMF / EtOH (1:1 / v: v) The solution was recrystallized to obtain a brownish-yellow solid, which was the acceptor compound (4.30 mmol), and the yield was 86.7%.
[0050] 2. Hg 2+ Preparation and detection of test paper
[0051] (1) Hg 2+ Preparation of detection test paper: the concentration of acceptor compound DMSO solution is 1.5×10 -3 mol L -1 Others are with embodiment 1.
[0052] (2) Test paper to detect Hg 2+ : with embodiment ...
Embodiment 3
[0054] 1. Synthesis of receptor compounds
[0055] (1) Synthesis of intermediate 5-(nitrophenyl)furan-2-carbaldehyde: same as in Example 1.
[0056] (2) Synthesis of acceptor compounds: Add about 1.1g (5mmol) of 5-(nitrophenyl)furan-2-carbaldehyde and about 1.0g (7.25mmol) of α-naphthylamine into a 50mL round bottom flask, dissolve in In 30mL hot EtOH, add 1mLAcOH as a catalyst, then reflux at 85°C for 4h, after cooling to room temperature, filter the brown precipitate with suction, wash the precipitate with hot EtOH three times, and finally recrystallize with DMF / EtOH solution to obtain a brown solid , which is the acceptor compound (ATS) (4.2mmol), yield 85.2%.
[0057] 2. Hg 2+ Preparation and detection of test paper
[0058] (1) Hg 2+ Preparation of detection test paper: the concentration of acceptor compound DMSO solution is 2.0×10 -3 mol L - 1. Others are the same as in Embodiment 1.
[0059] (2) Test paper to detect Hg 2+ : with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com