Preparation method for N-methylmorpholine
A technology of methylmorpholine and morpholine, applied in the field of preparation of organic compounds, to achieve the effects of cost reduction, low production cost and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, a kind of preparation method of N-methylmorpholine, carry out following steps successively:
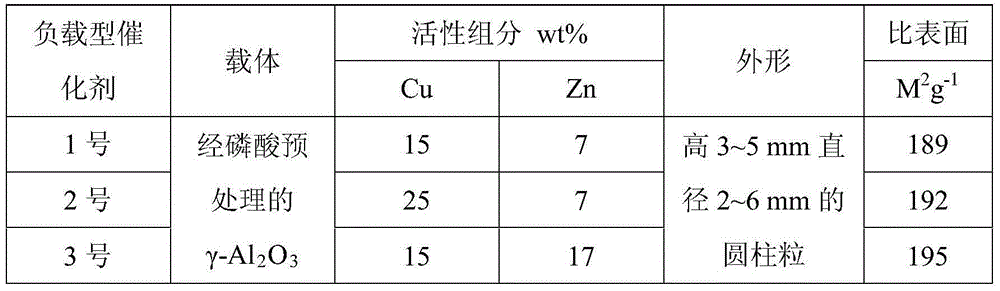
[0033] 1), preparation of supported catalyst:
[0034] a), the commercially available γ-Al 2 o 3 With a volume concentration of 5% H 3 PO 4 Soak the solution at 40-50°C for 60 minutes, filter out and dry, then bake at 400°C for 2 hours and 750°C for 6 hours to obtain the treated γ-Al 2 o 3 ,spare.
[0035] b) Add distilled water to 28.1g of copper nitrate and 13.3g of zinc nitrate solid (excluding crystal water) to make the volume to 50mL. After the copper nitrate and zinc nitrate are dissolved, 50mL of metal salt solution is obtained for later use.
[0036] With 1.2mL metal salt solution / 1.0gγ-Al 2 o 3 The ratio of the carrier, the γ-Al after the above treatment 2 o 3 Soak in the prepared solution for 36 hours; filter to obtain filtrate and catalyst.
[0037] c) The catalyst obtained after the above-mentioned filtration was dried at 60° C. for 2 hours ...
Embodiment 2
[0043] Embodiment 2, a kind of preparation method of N-methylmorpholine:
[0044] In step 2), the mol ratio of morpholine and methyl formate is changed to 1:0.9, the reaction temperature is controlled at 120°C, the hydrogen pressure is adjusted so that the reactor pressure remains at 0.15Mpa, and the raw material is 0.3g / h / mL catalyst The space velocity enters the catalyst bed layer to react. All the other are equal to embodiment 1.
[0045] The gas chromatographic analysis content of the obtained product is 90%, and the product yield is 95%.
Embodiment 3
[0046] Embodiment 3, a kind of preparation method of N-methylmorpholine:
[0047] In step 2), the molar ratio of morpholine and methyl formate is changed to 1:0.7, the reaction temperature is controlled at 125°C, the hydrogen pressure is adjusted so that the reactor pressure remains at 0.12MPa, and the raw material is 0.1g / h / mL catalyst The space velocity enters the catalyst bed layer to react. All the other are equal to embodiment 1.
[0048] The gas chromatographic analysis content of the obtained product is 97%, and the product yield is 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com