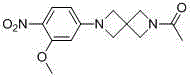Spiral ring or bridged ring containing pyrimidine compound
A technology of compounds and pyrimidines, applied in the field of compounds for the treatment of non-small cell lung cancer and its preparation, can solve the problem of low clinical drug tolerance dose, decreased affinity between inhibitors and EGFR, and inability to solve the clinical pressure of drug resistance, etc. problem, to achieve the effect of improving the stability of biological metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Example 1: N-(3-((2-((4-(6-acetyl-2,6-diazaspiro[3.3]heptane-2-yl)-2-methoxyphenyl) Amine)-5-(trifluoromethyl)pyrimidin-4-yl)amine)phenyl)acrylamide
[0103]
[0104] Step 1: tert-butyl (3-((2-chloro-5-(trifluoromethyl)pyrimidin-4-yl)amino)phenyl)carbamate
[0105]
[0106] tert-Butyl 3-aminobenzoate (4.8 g, 23 mmol) was dissolved in n-butanol (45 mL), then cooled to 0 o c. 2,4-Dichloro-5-(trifluoromethyl)-pyrimidine (5.0 g, 23 mmol) and DIPEA were sequentially added dropwise to the above solution. The resulting mixture is between 0-5 o C was stirred for one hour and then warmed to room temperature until the reaction was complete. The reaction solution was slurried with PE (45 mL) and filtered. The filter cake was washed with PE, collected and dried under vacuum to obtain a white solid (tert-butyl 3-((2-chloro-5-(trifluoromethyl)pyrimidin-4-yl)amino)phenyl)carbamate (4.434 g, yield rate: 49.6%). LC-MS: m / z 389 (M+H) + .
[0107] Step 2: N-(3-((2-Chloro-5...
Embodiment 2
[0126] Example 2: N-(3-((2-((4-(3-acetyl-3,8-diazabicyclo[3.2.1]octane-8-yl)-2-methoxybenzene base) amine)-5-(trifluoromethyl)pyrimidin-4-yl)amine)phenyl)acrylamide
[0127]
[0128] The synthetic method is as embodiment 1
[0129] 1 H NMR (400 MHz, DMSO-d 6 ) δ 10.17 (s, 1H), 8.61 (s, 1H), 8.26 (s, 1H), 8.09 (s, 1H), 7.74 (s, 1H), 7.44 (m 2H), 7.22 (s, 2H), 6.44 (m, 2H), 6.29 – 6.00 (m, 2H), 5.75 (d, J = 9.8 Hz, 1H), 4.22 (s, 2H), 3.91 (d, J = 12.3 Hz, 1H), 3.74 (s, 3H), 2.82 (d, J = 12.9 Hz, 1H), 1.97-1.90 (m, 4H), 1.87 (s, 2H), 1.75 (d, J= 11.1 Hz, 1H), 1.54 (d, J = 10.2 Hz, 1H), 1.39-1.33 (m, 1H). LC-MS: m / z 582 [M+H] + .
Embodiment 3
[0130] Example 3: N-(3-((2-((4-(4-acetyl-4,7-diazaspiro[2.5]octane-7-yl)-2-methoxyphenyl) Amine)-5-(trifluoromethyl)pyrimidin-4-yl)amine)phenyl)acrylamide
[0131]
[0132] The synthetic method is as embodiment 1
[0133] 1 H NMR (400 MHz, DMSO-d 6 ) δ 10.18 (s, 1H), 8.67 (s, 1H), 8.28 (s, 1H), 8.07 (s, 1H), 7.74 (s, 1H), 7.55 (d, J = 7.1 Hz, 1H), 7.48 (d, J = 8.7 Hz, 1H), 7.26 (t, J = 7.8 Hz, 1H), 7.14 (s, 1H), 6.54 (s, 1H), 6.47 – 6.35 (m, 1H), 6.26 (m, 1H), 6.13 (s, 1H), 5.76 (d, J = 10.0 Hz, 1H), 3.76 (s, 3H), 3.67 (d, J = 11.9 Hz, 2H), 3.08 (m, 2H), 2.93 (s, 2H), 2.08 (s, 3H), 1.1-0.68 (m, 4H). LC-MS: m / z 582 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com