Method for precipitating vanadium from high-aluminum vanadium-containing solution
A technology of vanadium precipitation and solution is applied in the field of hydrometallurgy, which can solve the problems of large consumption of ammonium salt and long reaction time, and achieve the effects of high vanadium precipitation rate and high purity of refined vanadium products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
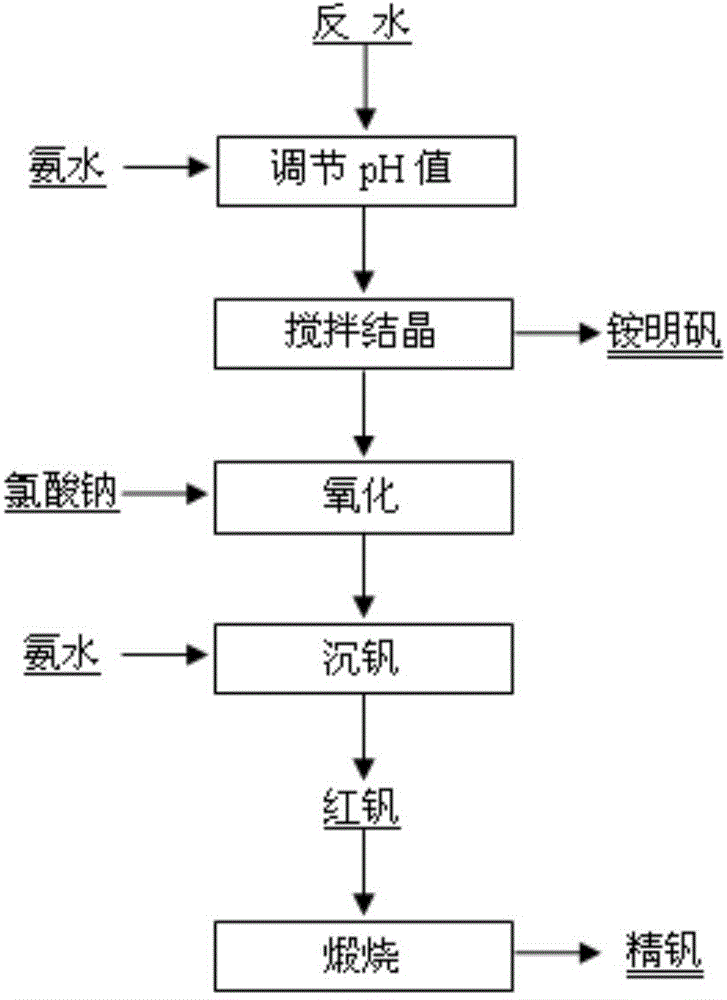
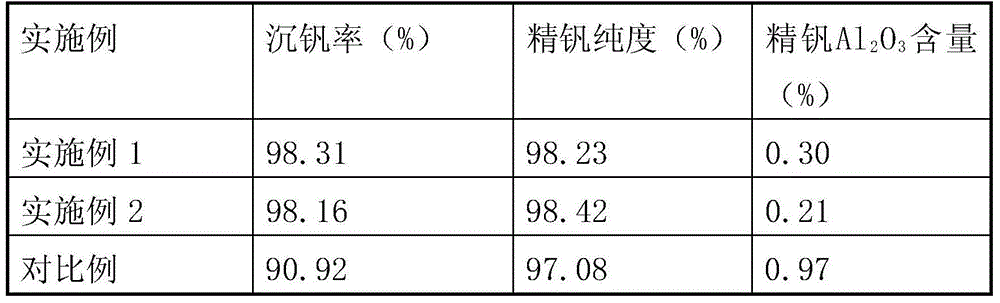
[0017] 160kg of stone coal combustion fly ash (vanadium-containing fly ash) in a certain place in Shaanxi was reversed by "acid leaching-extraction-stripping" and reversed water V 2 o 5 The concentration is 42.45g / L, Al 3+ The concentration is 11.68g / L. Add ammonia water to adjust the pH value to 1.8, stir and crystallize at 20°C for 4 hours to precipitate ammonium vanadium; add sodium chlorate to the crystallization tail liquid to oxidize low-valent vanadium ions, control the potential at 1080mV, and oxidize at 60°C for 1.0h; add 25% Adjust the pH value to 2.5 with ammonia water, stir to precipitate vanadium at 93°C for 2 hours, and filter to obtain red vanadium (cake); calcinate the red vanadium cake at 550°C for 2 hours to obtain refined vanadium. See Table 1 for the rate of vanadium precipitation and the purity of refined vanadium.
Embodiment 2
[0019] 1500g of stone coal-type vanadium ore in a certain place in Chongqing was reversed by "acid leaching-extraction-stripping" and the reversed water V 2 o 5 The concentration is 70.58g / L, Al 3+ The concentration is 13.60g / L. Add ammonia water to adjust the pH value to 1.9, stir and crystallize at 10°C for 2 hours to precipitate ammonium vanadium; add sodium chlorate to the crystallization tail liquid to oxidize low-valent vanadium ions, control the potential at 1060mV, and oxidize at 60°C for 1.0h; add 25% Ammonia water was used to adjust the pH value to 2.3, the vanadium was precipitated by stirring at 94°C for 2 hours, and red vanadium was obtained by filtration; the red vanadium was calcined at 550°C for 2 hours to obtain refined vanadium. See Table 1 for the rate of vanadium precipitation and the purity of refined vanadium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com