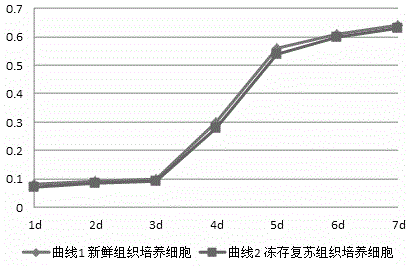
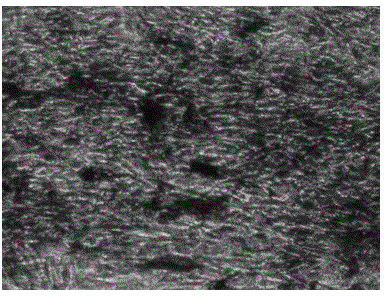
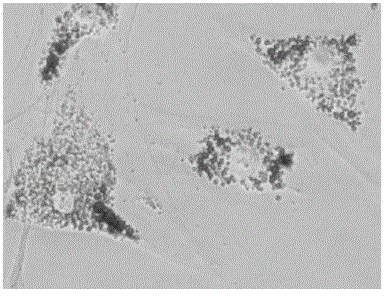
Cryopreservation protection liquid for Wharton jelly tissues of human umbilical cord and preparation and application of cryopreservation protection liquid
A technology for Wharton's jelly tissue and human umbilical cord, applied in the biological field, can solve the problems of long time and cost, affecting the freezing effect, unfavorable quality control, etc., and achieve the effects of less damage, low toxic and side effects, and good freezing effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0045] The present invention will be further described in detail below in conjunction with the accompanying drawings and specific embodiments.
[0046] It should be noted that the reagents used in the present invention are all prior art and may be commercially available products.
[0047] 1. Specific implementation
[0048] 1. Human umbilical cord Wharton's jelly
[0049] A cryopreservation solution for human umbilical cord Wharton's jelly tissue, its components and the volume fraction of each component are: 5-10% of permeable cryoprotectant, 1-5% of non-permeable cryoprotectant, serum replacement Product 10~20% and serum-free basal medium 70~80%.
[0050] During specific implementation, each component and the volume fraction of each component may be: 10% permeable cryoprotectant, 5% non-permeable cryoprotectant, 10% serum substitute and 75% serum-free basal medium.
[0051] More specifically, the components of human umbilical cord Wharton's jelly tissue and the volume ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com