Method for preparing bivalirudin freeze-dried powder needle preparation for injection or transfusion
A technology of bivalirudin and freeze-dried powder injection, which is applied in the field of biomedicine, can solve the problems of difficult to determine the formulation of the preparation, affect the quality of the preparation product, and complicate the preparation process, so as to achieve clear points of attention, promote aggregation and particle release, The effect of simple formula and process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The composition of the bivalirudin freeze-dried powder preparation for injection or infusion in this example is as follows:
[0052] Bivalirudin 63%
[0053] Low endotoxin mannitol 32%
[0054] Analytical Pure AR Grade Sodium Hydroxide 5.0%
[0055] A certain amount of water for injection after sterilization
[0056] Nitrogen half an atmosphere
[0057] Its preparation process is:
[0058] (1) Pretreatment: The bivalirudin raw material drug is in a freeze-dried powder state, and after analysis and detection, the content of impurities meets the USP requirements, and then refrigerated for later use;
[0059] (2) Preparation of neutralizing reagent: Weigh 0.8g of analytically pure AR grade NaOH solid and dilute it with sterilized water for injection to 100ml to obtain a 0.2mol / l analytically pure AR grade NaOH solution for later use;
[0060] (3) Buffer solution preparation: Weigh 600mg of endotoxin mannitol, add 10ml of sterilized water for injection, about a dose of 2...
Embodiment 2
[0068] In this example, the bivalirudin freeze-dried powder preparation consists of the following components:
[0069] Bivalirudin 63%
[0070] Low endotoxin mannitol 32%
[0071] Analytical Pure AR Grade Sodium Hydroxide 5.0%
[0072] Water for injection after sterilization A certain amount
[0073] half an atmosphere of nitrogen
[0074] Its preparation process is:
[0075] (1) Pretreatment: The bivalirudin raw material drug is in a freeze-dried powder state, and after analysis and detection, the content of impurities meets the USP requirements, and then refrigerated for later use;
[0076] (2) Preparation of neutralizing reagent: Weigh 0.8g of analytically pure AR grade NaOH solid and dilute it with sterilized water for injection to 100ml to obtain a 0.2mol / l analytically pure AR grade NaOH solution for later use;
[0077] (3): Weigh 375mg of endotoxin mannitol, add 5ml of sterilized water for injection, about a dose of 2ml of water, dissolve, and prepare 6% endotoxin ...
Embodiment 3-6
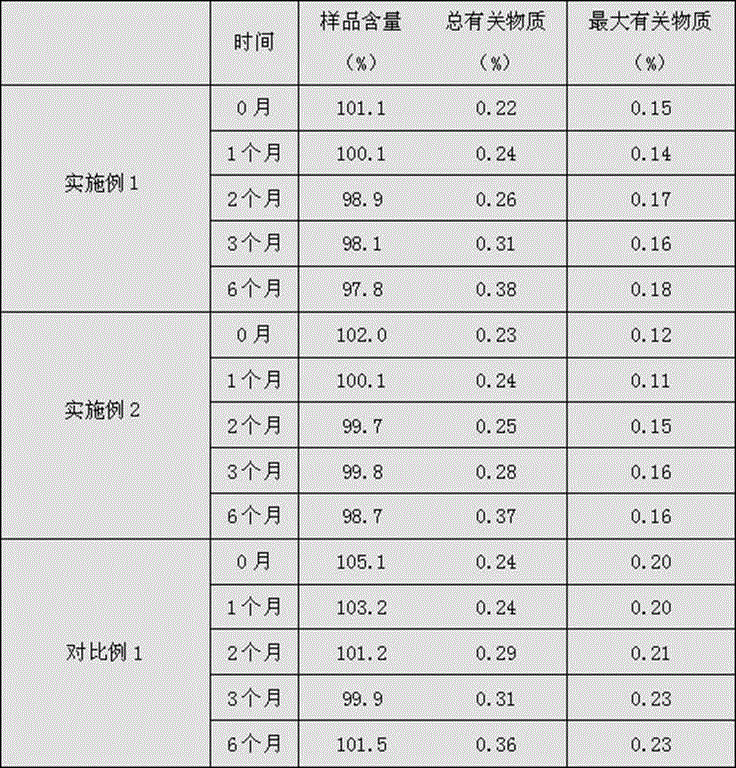
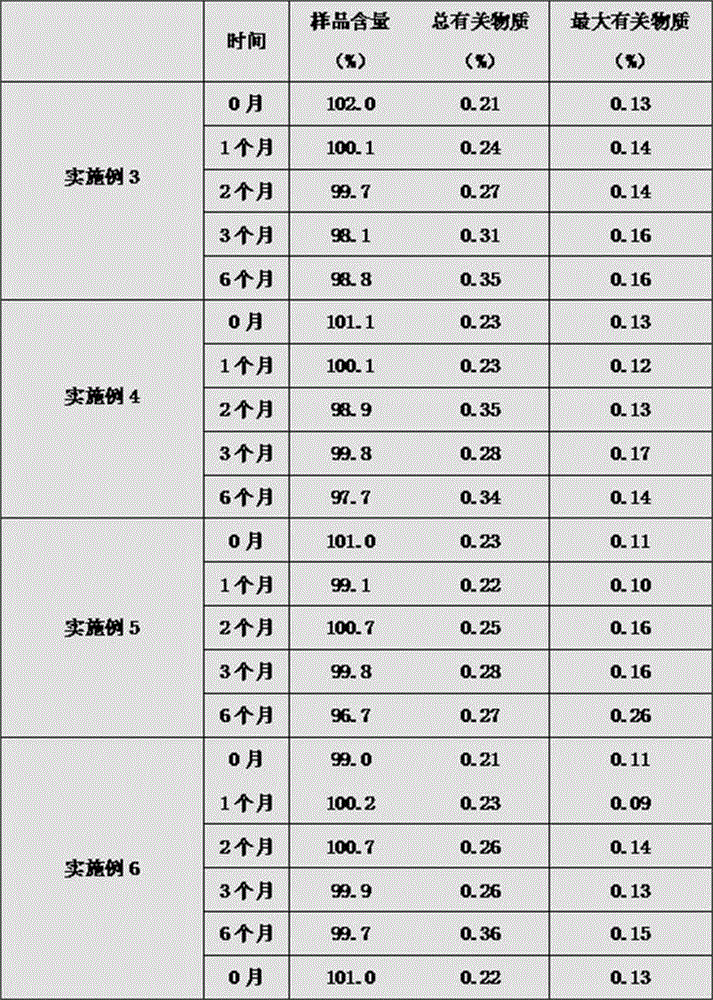
[0091] The preparation method of the bivalirudin freeze-dried powder preparation in this example 3-6 is the same as that of Examples 1 and 2, wherein the ratio of bivalirudin, endotoxin mannitol and analytically pure AR grade sodium hydroxide is shown in the table below 4. See Table 5 below for freeze-drying parameters and Table 6 below for performance parameters.
[0092] Example Bivalirudin endotoxin mannitol Analytical Pure AR Grade Sodium Hydroxide Example 3 60% 35% 5% Example 4 70% 25% 5% Example 5 63.5% 30% 6.5% Example 6 65% 29.2% 5.8%
[0093] step Set temperature °C Decrease / heat up time min duration min vacuum mtorr pre-freeze -20 75 60 none Frozen -45 45 180 none a sublimation -10 120 390 120 secondary sublimation 0 90 450 120 triple sublimation 12.5 120 120 120 once dry 25 45 300 120 secondary drying 30 60 330 0 Finish 0 0 0 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com