Method for removing volatile halocarbons in environment through chemical conversion
A chemical conversion, halogenated hydrocarbon technology, applied in the field of environmental engineering, can solve the problems of ineffective contact of pollutants, slow oxidation reaction, inability to carry current, etc., to achieve no secondary pollution, short repair period, and complete conversion. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
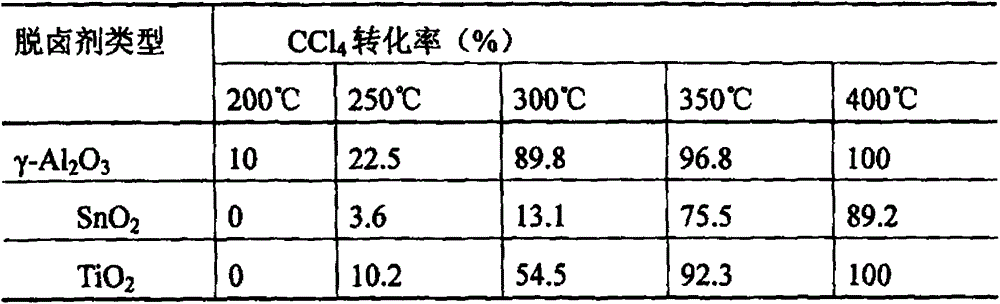
[0045] Metal oxides to CCl at different temperatures 4 The removal effect of
[0046] metal oxide (Al 2 o 3 , SnO 2 、TiO 2 etc.) Activate in a muffle furnace at 500°C for 4h, take 0.8g and put it into a fixed bed reactor, and feed 1010ppm of CCl 4 / N 2 Gas, gas flow rate 37.7ml / min, heating reaction at 200℃~400℃, CCl at different temperatures 4 The conversion rate is shown in Table 1.
[0047] Table 1 Metal oxides to CCl at different temperatures 4 The removal effect of
[0048]
Embodiment 2
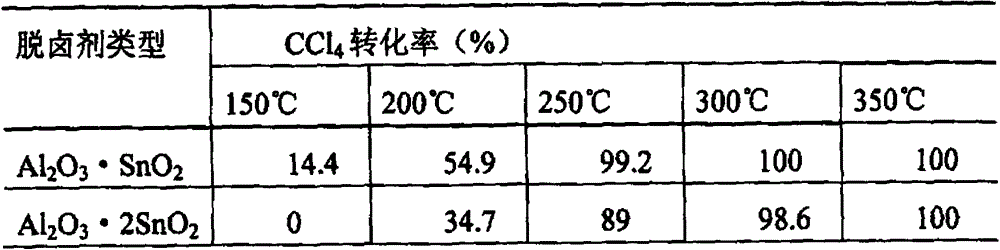
[0050] Composite metal oxides to CCl 4 The removal effect of
[0051] Mixed metal metal oxide Al 2 o 3 ·SnO 2 and Al 2 o 3 2SnO 2 Activate in a muffle furnace at 500°C for 4h, take 0.8g into a fixed bed reactor, and feed 1010ppm of CCl 4 / N 2 Gas, gas flow rate 37.7ml / min, heating reaction at 150℃~350℃, CCl at different temperatures 4 The conversion rate is shown in Table 2.
[0052] Table 2 Composite metal oxides to CCl 4 The removal effect of
[0053]
Embodiment 3
[0055] Type A molecular sieve to CCl 4 The removal effect of
[0056] Activate type A molecular sieves (type 3A, type 4A, type 5A) in a muffle furnace at 500°C for 4h, take 0.8g and put it into a fixed bed reactor, and feed 1010ppm of CCl 4 / N 2 Gas, gas flow rate 37.7ml / min, heating reaction at 150℃~300℃, CCl at different temperatures 4 The conversion rate is shown in Table 3.
[0057] Table 3 Type A molecular sieve to CCl 4 The removal effect of
[0058]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com