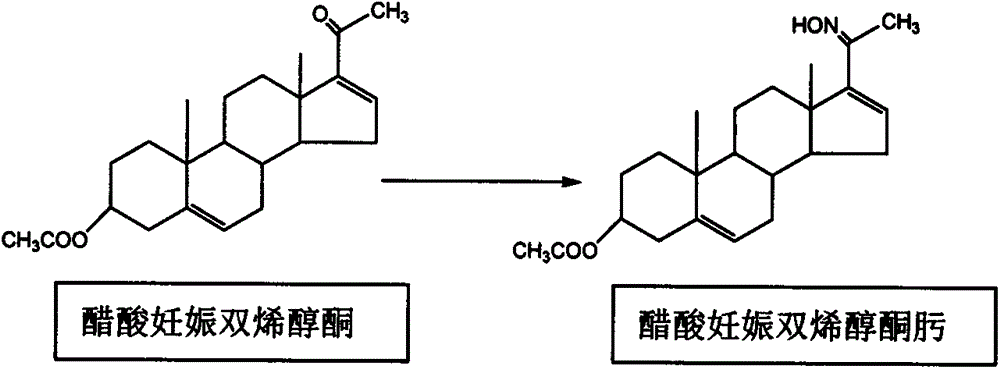
Production method of 16-dehydropregnenolone acetate oxime
A technology of acetic acid pregnant dienolone and dienolketoxime, which is applied in the field of production technology of steroid hormone drug intermediates, and achieves the effects of high product quality, simple operation and good reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0042] Embodiments of the invention:
[0043]In 50 liters of reactors, add 7 kilograms of acetic acid pregnant dienolones, 3.5 kilograms of pyridine, 25 kilograms of solvent methanol, 8 kilograms of solvent dichloroethanes, add 1.6 kilograms of hydroxylamine hydrochloride, 0.007 kilograms of N, N from the solid feeding port - lutidine, stirring and heating until the temperature of the reaction kettle reaches reflux, keeping the reflux reaction for 1 hour and 30 minutes, sampling and detecting the content of acetic acid pregnant dienolone. Stop heating until the content of dienolone in acetic acid pregnancy is below 0.5%, pass through cooling water to cool to about 10°C, stir at about 10°C for 30 minutes, filter, and rinse the filter cake with 2-5 kg of reaction mixed solvent Twice, the filter cake was dried to obtain 7 kilograms, and the liquid chromatography standard sample method detection content was 99%. The molar yield was 95.8%. The filtrate was dried and weighed wit...
specific Embodiment 2
[0065] In 50 liters of reactors, add 7 kilograms of acetic acid pregnant dienolones, 3.5 kilograms of pyridine, 25 kilograms of solvent ethanol, 8 kilograms of solvent dichloroethanes, add 1.6 kilograms of hydroxylamine hydrochloride, 0.007 kilograms of N, N from the solid feeding port - lutidine, stirring and heating until the temperature of the reaction kettle reaches reflux, keeping the reflux reaction for 1 hour and 30 minutes, sampling and detecting the content of acetic acid pregnant dienolone. Stop heating until the content of dienolone in acetic acid pregnancy is below 0.5%, pass through cooling water to cool to about 10°C, stir at about 10°C for 30 minutes, filter, and rinse the filter cake with 2-5 kg of reaction mixed solvent Twice, the filter cake was dried to obtain 7 kilograms, and the liquid chromatography standard sample method detection content was 98%. The molar yield was 94.1%. The filtrate was dried and weighed with anhydrous sodium sulfate, and the rati...
specific Embodiment 3
[0076] In 50 liters of reactors, add 7 kilograms of acetic acid pregnant dienolones, 3.5 kilograms of pyridine, 25 kilograms of solvent ethanol, 5 kilograms of solvent chloroforms, add 1.6 kilograms of hydroxylamine hydrochloride, 0.007 kilograms of N, N- lutidine, stirring and heating until the temperature of the reaction kettle reaches reflux, keeping the reflux reaction for 1 hour and 30 minutes, sampling and detecting the content of acetic acid pregnant dienolone. Stop heating until the content of dienolone in acetic acid pregnancy is below 0.5%, pass through cooling water to cool to about 10°C, stir at about 10°C for 30 minutes, filter, and rinse the filter cake with 2-5 kg of reaction mixed solvent Twice, the filter cake was dried to obtain 7.1 kg, and the detection content by the liquid chromatography standard sample method was 98.5%. The molar yield was 95.9%. The filtrate was dried and weighed with anhydrous sodium sulfate, and the ratio of ethanol and chloroform w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com