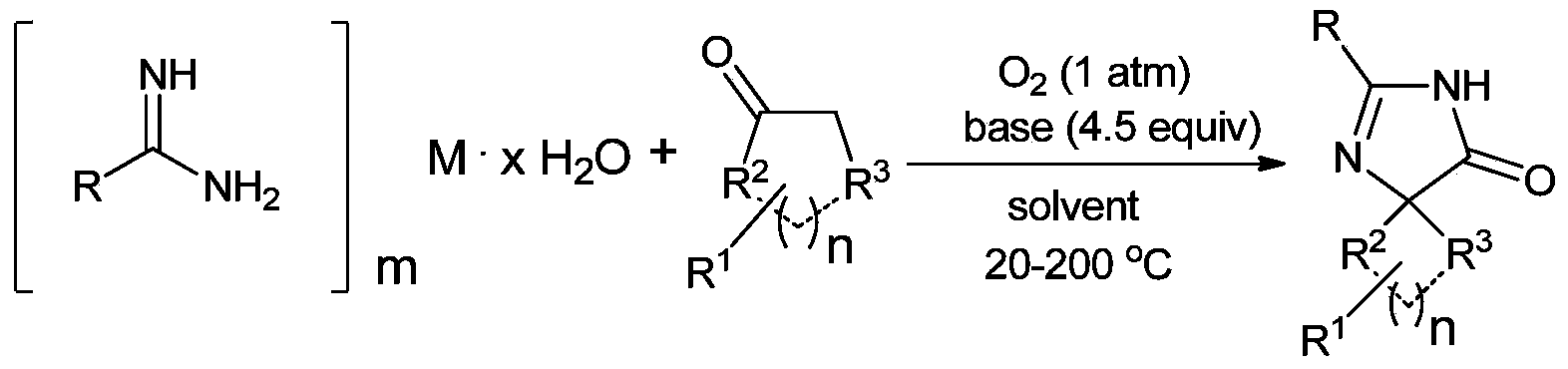
4, 4-disubstituted-4, 5-dihydro-1H-imidazole-5-one and derivatives thereof as well as synthesis method thereof
A synthetic method, -1H technology, applied in the direction of organic chemistry, etc., to achieve the effects of excellent chemical properties, reduced environmental pollution, and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
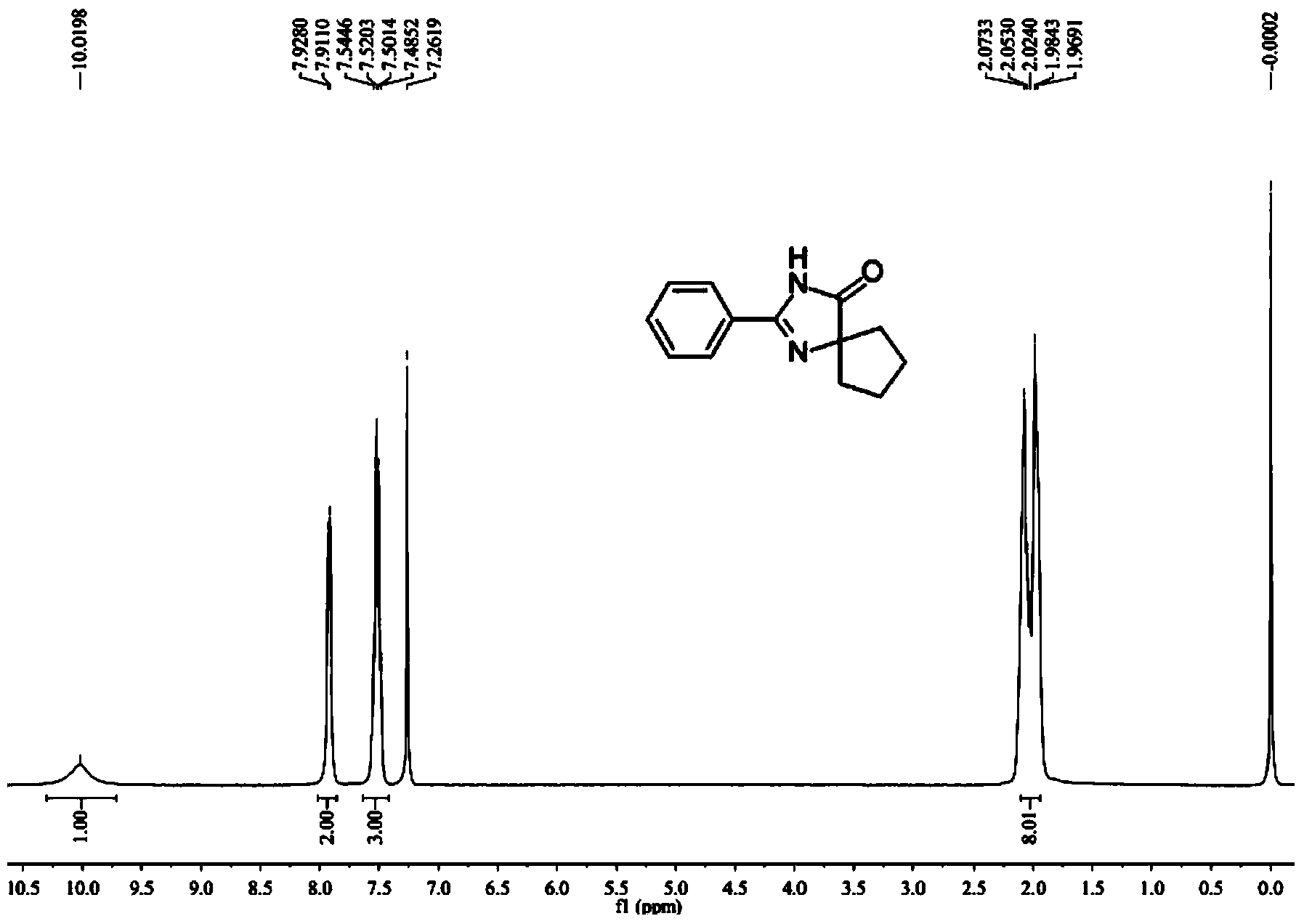
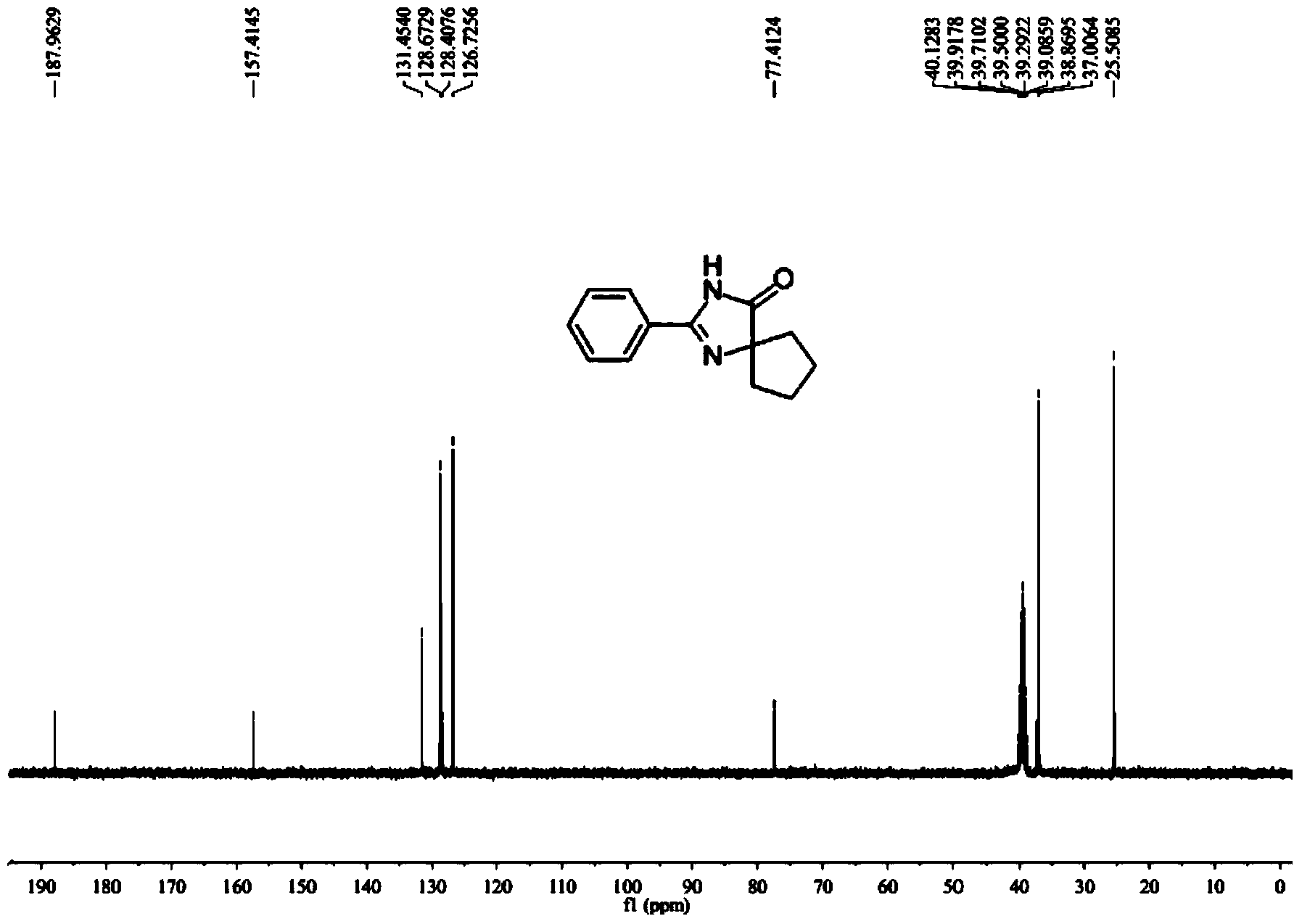
[0106] Synthesis of Example 12-phenyl-1,3-diazaspiro[4,4]non-1-en-4-one
[0107]
[0108] Take a reaction tube, add 0.9mmol (36mg) sodium hydroxide, 0.2mmol (35.6mg) benzamidine hydrochloride monohydrate, 0.3mmol (31.2μL) cyclohexanone, 0.8mL pyridine under oxygen protection, 80℃ The reaction was carried out for 24 hours, and 36.8 mg of pure product was obtained by conventional treatment, with a yield of 86%.
[0109] The NMR and mass spectrometry data of embodiment 1 product are as follows:
[0110] 1 H NMR (400MHz, CDCl 3 ,ppm)δ10.02(br,1H),7.92(d,J=6.8Hz,2H),7.54-7.49(m,3H),2.07-1.97(m,8H); 13 C NMR (100MHz, DMSO-d 6 , ppm) δ187.8, 157.0, 131.0, 128.2, 127.9, 126.2, 76.9, 36.5, 25.0; MS (EI) m / z (%) 214, 171, 104 (100), 83, 54.
Embodiment 22
[0111] Synthesis of Example 22-(p-methylphenyl)-1,3-diazaspiro[4,4]non-1-en-4-one
[0112]
[0113] Take a reaction tube, add 0.9mmol (36mg) sodium hydroxide, 0.2mmol (34.1mg) 4-methylbenzamidine hydrochloride, 0.3mmol (31.2μL) cyclohexanone, 0.8mL quinoline under oxygen protection, React at 40°C for 48 hours. Conventional treatment yielded 39.7 mg of pure product with a yield of 87%.
Embodiment 2
[0114] The NMR and mass spectrometry data of embodiment 2 product are as follows:
[0115] 1 H NMR (500MHz, CDCl 3 ,ppm)δ10.14(br,1H),7.80(d,J=8.0Hz,2H),7.31(d,J=8.1Hz,2H),2.42(s,3H),2.07-1.94(m,8H ); 13 C NMR (125MHz, DMSO-d 6 , ppm) δ187.3, 156.1, 135.7, 128.3, 128.0, 126.2, 77.0, 36.5, 25.0, 20.5; MS (EI) m / z (%) 228, 185, 118 (100), 83, 65; HRMS calcd.for: C 14 h 17 ON 2 [M+H] + 229.1335, found 229.1333.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com