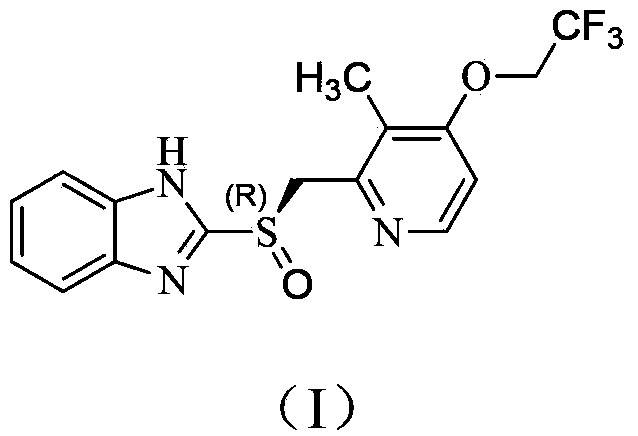
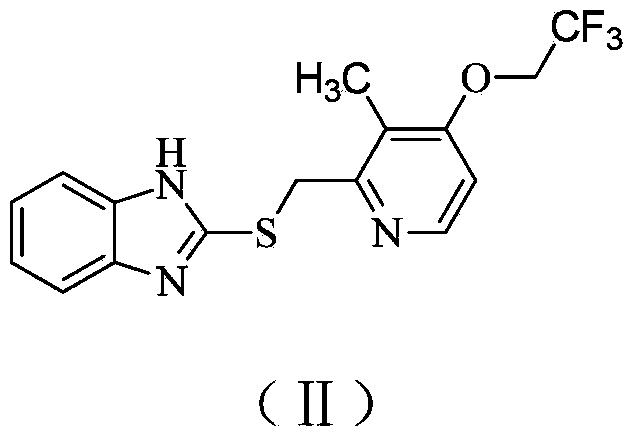
Method for antipodal selective synthesis of (R)-lansoprazole
A technology of dexlansoprazole and enantioselectivity, which is applied in the field of chemical synthesis drug preparation, can solve the problems of low optical purity of sulfoxide, difficult removal of sulfone, and increased post-processing difficulty, etc., to achieve optical purity and chemical purity of the product High, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1 dexlansoprazole
[0038] In a 20L reactor, lansoprazole sulfide (1.77kg, 5.0mol) was suspended in toluene (8.8L), and L-(+)-diethyl tartrate (412g, 2.0mol) and water (9.0g, 0.5mol), heated at 50-60°C for 30 minutes. Tetraisopropyl titanate (284 g, 1.0 mol) was added dropwise, and the reaction was continued at 55-60°C for 50 minutes. Stop heating, cool to below 30°C, add diisopropylethylamine (194g, 1.5mol), continue cooling to below 10°C, then slowly add cumene hydroperoxide (78%, 1.37kg, 7.0mol ), react at 13-18°C for 3 hours. According to chromatographic analysis, sulfide is 1.6%, sulfone is 1.4%, sulfoxide is 96.8%, and ee value is 97.5%.
[0039] Na for system 2 S 2 o 3 Aqueous solution (30%, 3 L) was quenched and the layers were separated. Transfer the organic layer into a 50L reaction kettle, add water (1.7L), n-heptane (5.3L), tert-butyl methyl ether (7.0L), n-heptane (10.6L) dropwise at room temperature, and use Lower the ...
Embodiment 2
[0040] The preparation of embodiment 2 dexlansoprazole
[0041] In a 500mL three-necked flask, suspend lansoprazole sulfide (35.3g, 100mmol) in toluene (180mL), add L-(+)-diethyl tartrate (12.4g, 60mmol) and water (0.29g, 16mmol) , Heat the reaction at 50-60°C for 30 minutes. Tetraisopropyl titanate (5.7 g, 20 mmol) was added dropwise, and the reaction was continued under heating at 55-60°C for 1 hour. Stop heating, cool to below 30°C, add diisopropylethylamine (3.9g, 30mmol), continue cooling to below 10°C, then slowly add cumene hydroperoxide (78%, 25.4g, 130mmol) dropwise , 15-25°C for 3 hours. According to chromatographic analysis, sulfide is 1.9%, sulfone is 1.6%, sulfoxide is 96.2%, and ee value is 96.5%.
[0042] Refer to Example 1 for subsequent steps.
Embodiment 3
[0043] The preparation of embodiment 3 dexlansoprazole
[0044] In a 500mL three-necked flask, lansoprazole sulfide (35.3g, 100mmol) was suspended in toluene (180mL), added L-diisopropyl tartrate (10.3g, 50mmol) and water (0.15g, 8mmol), 50- The reaction was heated at 60°C for 30 minutes. Tetraisopropyl titanate (5.7 g, 20 mmol) was added dropwise, and the reaction was continued at 55-60° C. for 50 minutes. Stop heating, cool to below 30°C, add diisopropylethylamine (3.9g, 30mmol), continue cooling to 5°C, then slowly add cumene hydroperoxide (78%, 29.3g, 150mmol) dropwise, React at 10-15°C for 3 hours. According to chromatographic analysis, sulfide 1.5%, sulfone 1.5%, sulfoxide 96.5%, ee value 96.8%.
[0045] Refer to Example 1 for subsequent steps.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com