Trans-o-hydroxy stilbene derivative as well as preparation method and application thereof
A technology of o-hydroxystilbene and derivatives, which is applied in the field of preparation of trans-o-hydroxystilbene derivatives, can solve the problems of low yield, harsh reaction conditions, poor cis-trans stereoselectivity and the like, and achieves yield. The effect of high rate, short response time and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] In a 5mL single-port reaction flask, add 60mg (0.3mmol) 5-bromo-o-hydroxystyrene (1.0eq), 64mg3,5-dimethylphenylboronic acid, [Cp*RhCl 2 ] 2 3.7 mg (2%), CuOAc 120 mg (2.0 eq). Add 3 mL of methanol and stir at room temperature. After about 2 hours, TLC detected that the reaction was complete, and about 500 mg of silica gel was added, the solvent was evaporated, solidified, and the solid was loaded as a sample, and separated by column chromatography using petroleum ether and ethyl acetate as eluents. 52 mg of the product was obtained with a yield of 86%. The reaction formula is as follows:
[0046]
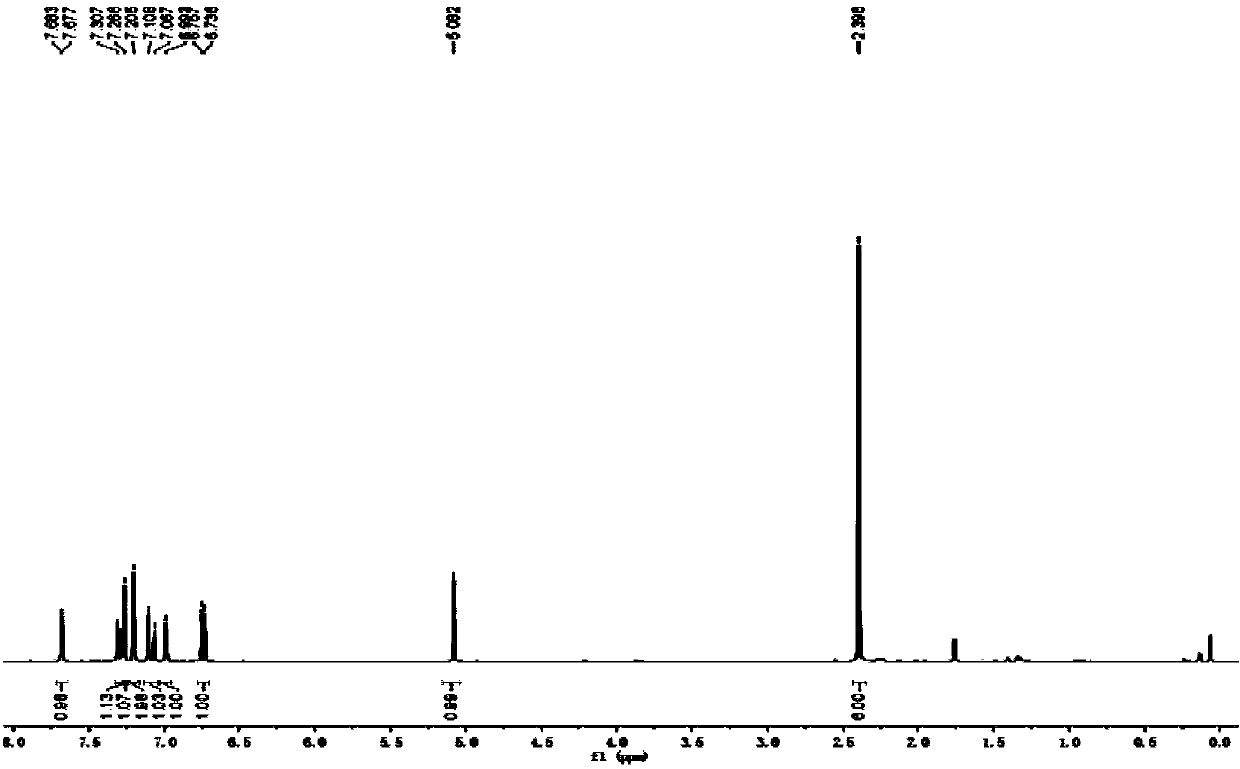
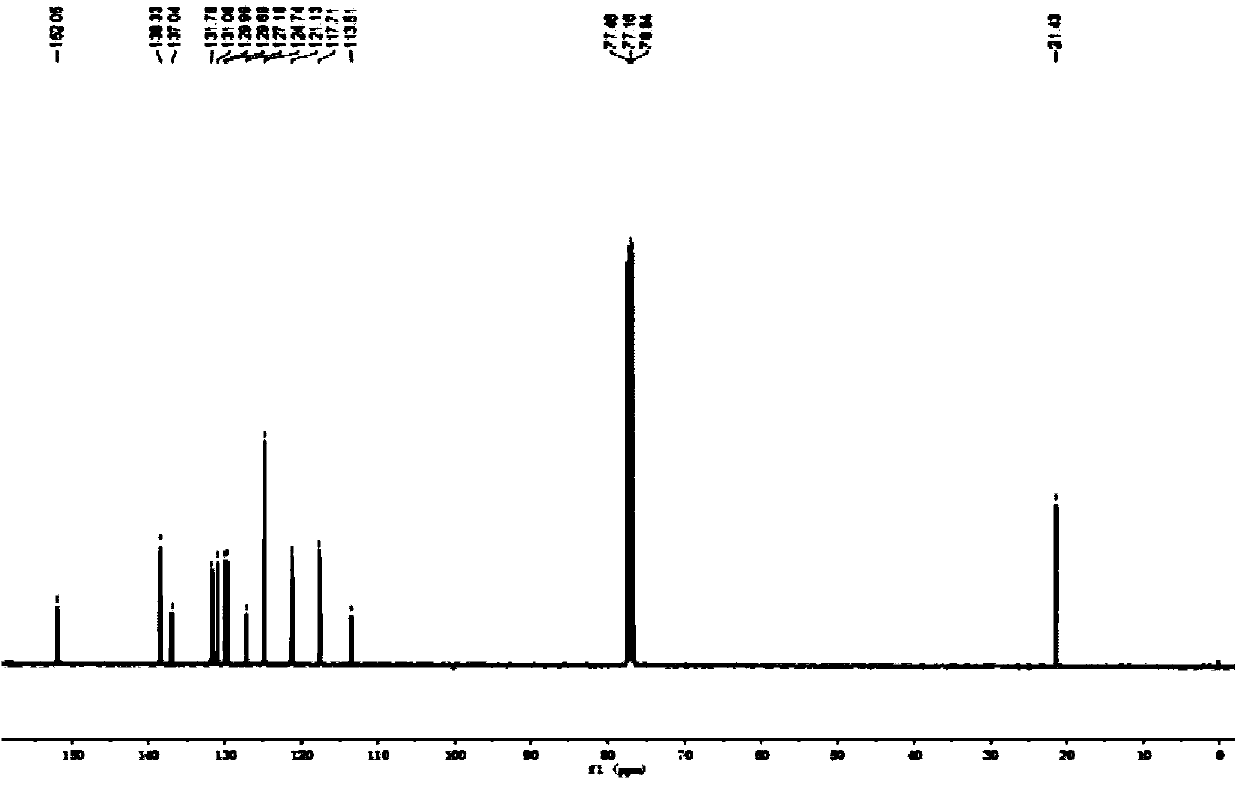
[0047] The physical properties and spectral data of the product are as follows: white solid; melting point: 90-92°C; 1 H NMR (CDCl 3 ,400MHz),δ:7.68(d,J=2.4Hz,1H),7.31-7.26(m,2H),7.20(s,2H),7.09(d,J=16.4Hz,1H),6.99(s, 1H), 6.75(d, J=8.4Hz, 1H), 5.08(s, 1H), 2.40(s, 6H); 13 C NMR (CDCl 3 ,100MHz), δ:152.1,138.3,137.0,131.8,131.1,130.0,129.7,127.2.124.7,121.1,117.7,...
Embodiment 2
[0049] In a 5mL single-port reaction flask, add 30mg (0.2mmol) 4-methoxy-o-hydroxystyrene (1.0eq), 89mg3,4,5-trimethylphenylboronic acid, [Cp*RhCl 2 ] 2 2.48 mg (2%), CuOAc 80 mg (2.0 eq). Add 2 mL of methanol and stir at room temperature. After about 2 hours, TLC detected that the reaction was complete, and about 500 mg of silica gel was added, the solvent was evaporated, solidified, and the solid was loaded as a sample, and separated by column chromatography using petroleum ether and ethyl acetate as eluents. 38 mg of the product was obtained with a yield of 60%. The reaction formula is as follows:
[0050]
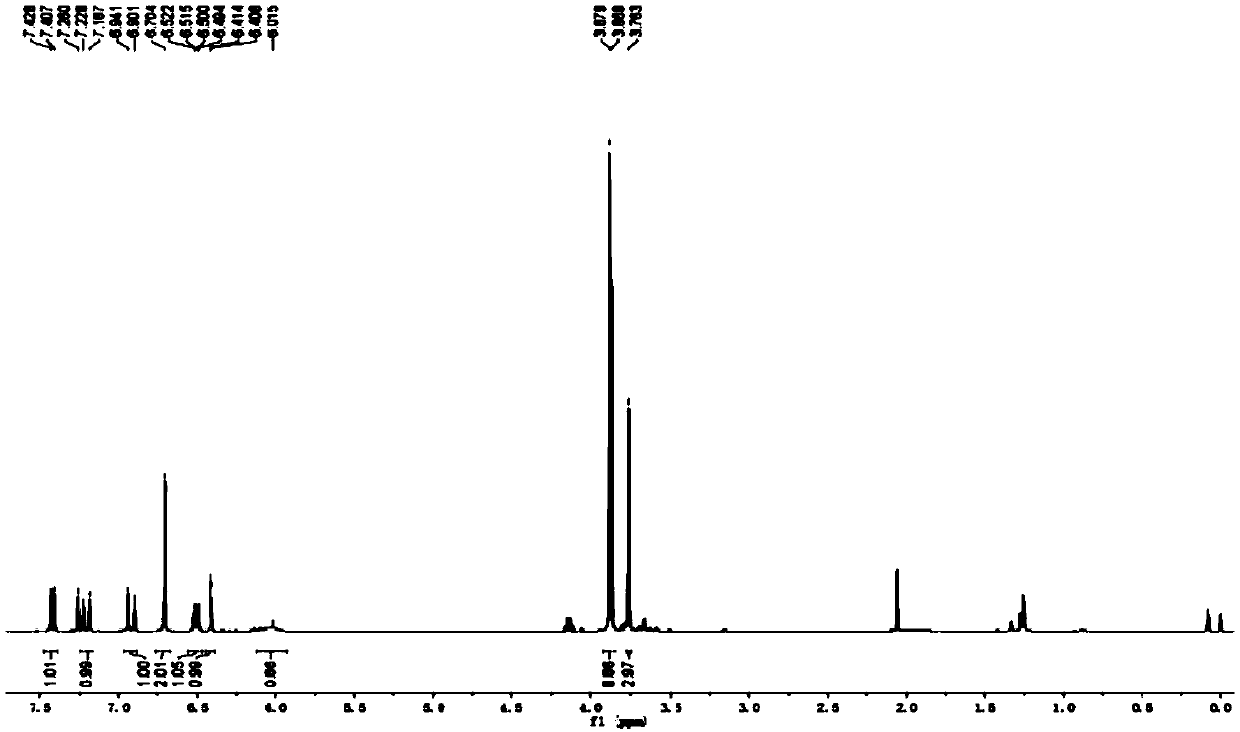
[0051] The physical properties and spectrogram data of the product are as follows: Oil; 1 H NMR (CDCl 3 ,400MHz),δ:7.42(d,J=8.4Hz,1H),7.21(d,J=16.4Hz,1H),6.92(d,J=16.0Hz,1H),6.70(s,2H),6.51 (dd, J 1 =8.8Hz,J 2 =2.8Hz,1H),6.41(d,J=2.4Hz,1H),6.02(br,s,1H),3.88(s,6H),3.87(s,3H),3.76(s,3H); 13 C NMR (CDCl 3 ,100MHz), δ:160.3,154.5,153.4,137.4,134.1,128.0,127.7,...
Embodiment 3~23
[0054] The preparation methods of Examples 3-23 are the same as Example 1, except that the two raw materials are replaced with other similar structures, and the structures of the obtained products are listed in Table 1.
[0055] The product structure of embodiment 3~23
[0056]
[0057]
[0058]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap