blys antagonistic peptide ss12, fusion protein ss12‑fc containing the antagonistic peptide and gene
A fusion protein and antagonistic peptide technology, applied in the field of biomedicine, can solve problems such as low efficiency, unstable peptides, and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Structural information on the interaction between BLyS and its receptors and the design of novel antagonistic peptides targeting BLyS
[0058] The interaction mode between BLyS and its receptor was explored by computer-aided molecular docking and dynamic simulation, and the key amino acid residues for mutual recognition between BLyS and its receptor were analyzed by means of computer graphics and distance geometry.
[0059] Using computer-aided molecular design and high-throughput virtual screening technology to de novo design short peptides that can simulate the conformation of key amino acids in receptors, and then theoretically evaluate the role of BLyS and antagonistic peptides by means of de novo construction, molecular docking, and kinetic simulation; use computer graphics Biochemical techniques, distance geometry, and intermolecular hydrogen bonding interactions were used to evaluate potential biological effects of antagonistic peptides. After theoretical screeni...
Embodiment 2
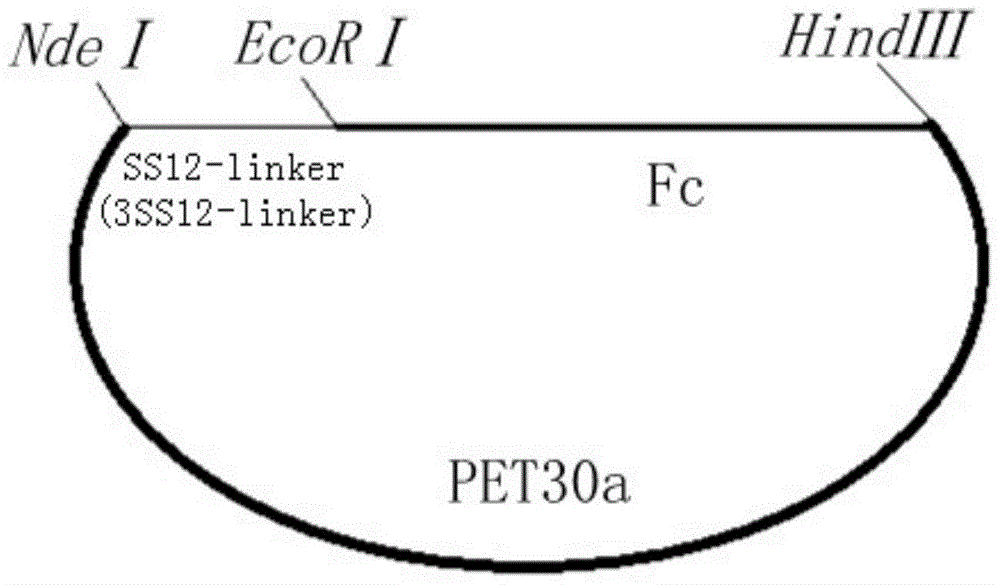
[0061] Plasmids SS12-Fc-pET30a and 3SS12-Fc-pET30a containing SS12-Fc and 3SS12-Fc fusion protein genes were constructed respectively ( figure 1 ).
[0062] 2.1 The gene encoding BLyS antagonistic peptide SS12 was constructed by the following method: According to the amino acid sequence of the antagonistic peptide SS12, we designed two DNA primers encoding short peptides, and ordered synthesis through the company.
[0063] The SS12 upstream primer is shown in SEQ ID NO.5, and the SS12 downstream primer is shown in SEQ ID NO.6
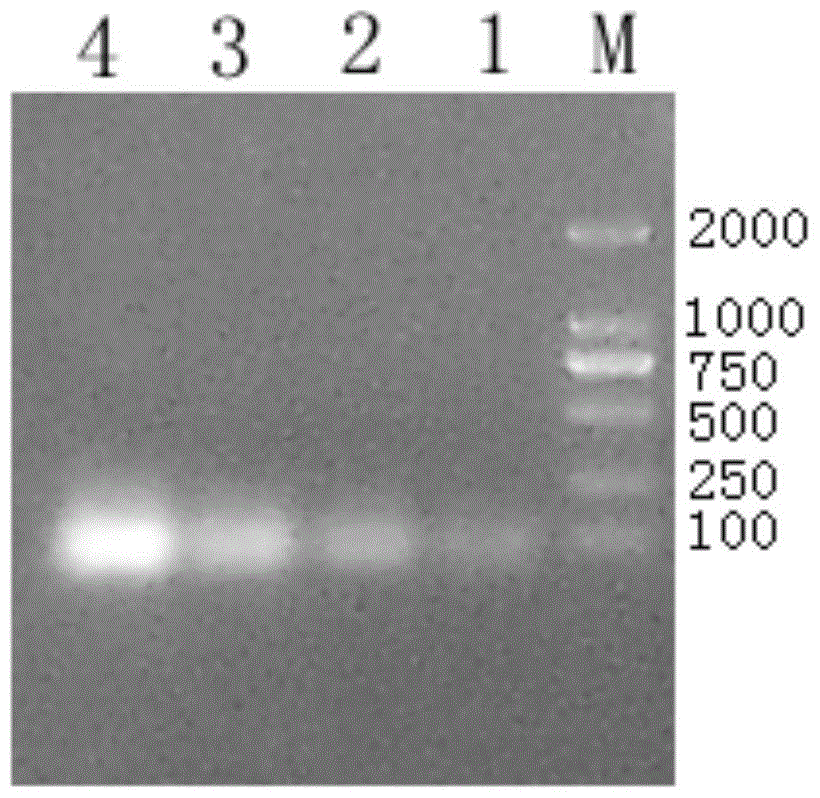
[0064] The synthetic SS12 DNA primer was dissolved in 200 μl of 10 mM Tris-Hcl (pH 8.5) (final concentration 16 pmol / μl). Take 20 μl of the positive and negative strands of the SS12 gene, mix them in EP tubes, boil in a water bath at 100°C for 3 minutes, and gradually cool down to room temperature. Use 2% agarose gel electrophoresis to observe the annealing result. The results of electrophoresis showed that there was an obvious band at 65bp ( figur...
Embodiment 3
[0078] Induction and expression of SS12-Fc and 3SS12-Fc fusion proteins
[0079] The correctly sequenced recombinant plasmids SS12-Fc-pET30a and 3SS12-Fc-pET30a were respectively transformed into the expression host strain E. coli. Under the condition of induction, the target protein can be induced. Select high-expression strains for mass expression, purify by protein A gel affinity chromatography, collect the eluate, dialyze in 1×PBS, measure and calculate the SS12-Fc protein concentration with a UV spectrophotometer to 700ng / μl, 3SS12 - Fc protein concentration is 1400 ng / μl. Get the target protein after dialysis and carry out SDS-PAGE electrophoresis, find, the size of SS12-Fc and 3SS12-Fc protein is respectively 25.4kDa and 28.6kDa, and SS12-Fc and 3SS12-Fc size are consistent, and have higher purity (see Figure 8 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com