Glutamine synthetase high-efficiency expression vector with dual expression cassettes
A technology of glutamine and expression vectors, which is applied in the field of biopharmaceutical genetic engineering expression, can solve problems such as unsuitable for uniform expression level, unsuitable for high-efficiency protein expression, and large differences in protein expression levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1, p327.7 expression vector whole gene synthesis and functional elements
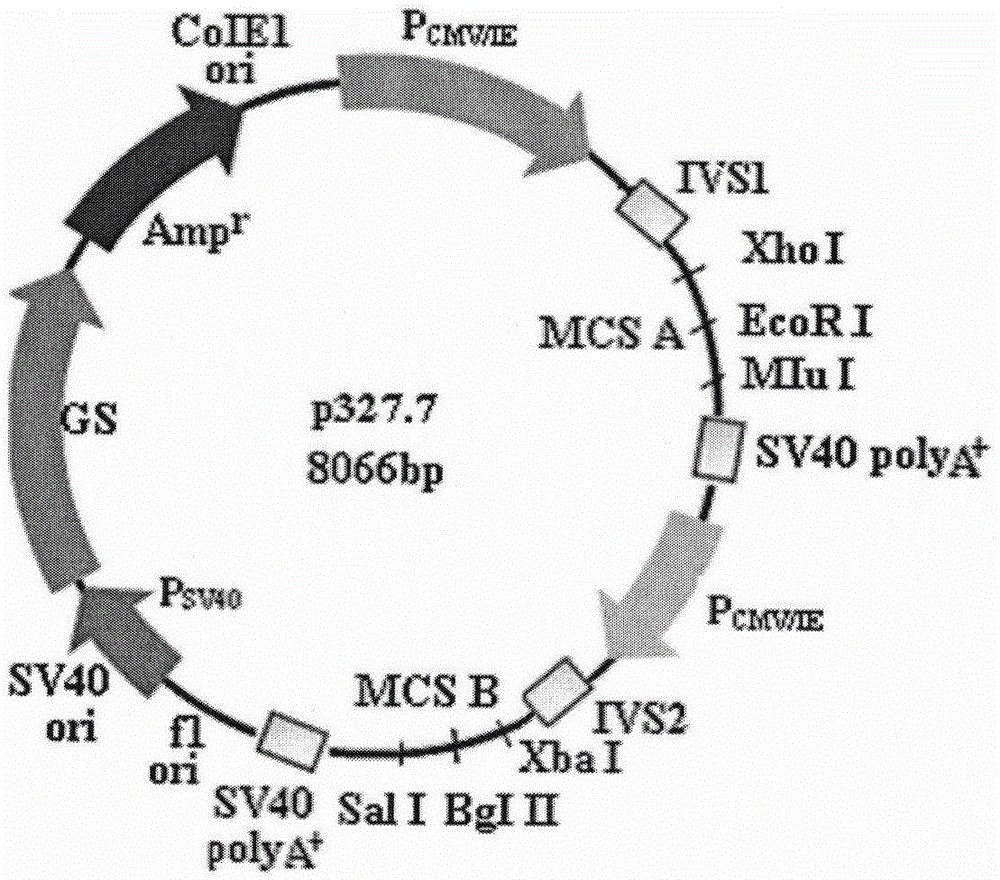
[0014] Entrusted Shanghai Jierui Bioengineering Co., Ltd. to synthesize the whole gene of p327.7 expression vector, 8066bp, and the schematic diagram of its vector structure is as follows figure 1 As shown, the sequence is shown in SEQ ID NO:1.
[0015] 1. The first expression cassette element
[0016] 1. CMV immediate enhancer (1-659bp), as shown in SEQ ID NO:2.
[0017] 2. CMV promoter (669-750bp), as shown in SEQ ID NO:3.
[0018] 3. T7 RNA polymerase promoter (1067-1085bp)
[0019] TAATACGACTCACTATAGG
[0020] 4. Multiple cloning site A (1091-1125): XhoI-EcoRI-MluI,
[0021] CTCGAGTCTTGATAGCACCTATTGAATTCACGCGT
[0022] 5. SV40 poly A signal 1 (1150-1353bp), as shown in SEQ ID NO:4.
[0023] 2. The second expression cassette element
[0024] 1. CMV immediate enhancer 2 (1664-2322bp), as shown in SEQ ID NO:5.
[0025] 2. CMV immediate early promoter 2 (2325-2405bp), as shown in...
Embodiment 2
[0043] Example 2, construction of expression vector for anti-Her2 / neu humanized antibody BY01
[0044] Entrusted Shanghai Jierui Bioengineering Co., Ltd. to synthesize the gene encoding the L chain of the anti-Her2 / neu humanized antibody, as shown in SEQ ID NO:13. Cloned into p327.7 expression vector after XhoI and EcoRI double digestion, named p327.7 / L:
[0045] The underlined parts are the restriction sites of XhoI and EcoRI respectively, and start and stop codons, respectively.
[0046] Also commissioned Shanghai Jierui Bioengineering Co., Ltd. to synthesize the gene encoding the H chain of the anti-Her2 / neu humanized antibody, as shown in SEQ ID NO:14. It was cloned into the p327.7 / L expression vector after XbaI- and SalI double digestion, and named as p327.7 / HER2 expression vector.
[0047] Wherein the underlined parts are respectively the enzyme cutting sites of XbaI and SalI, and start and stop codons
[0048] Example 2, Screening of Anti-Her2 / neu Humanized B...
Embodiment 3
[0050] Example 3, Expression and preliminary purification of humanized BY01 antibody
[0051] The BY01 cells highly expressing the humanized antibody were cultured in serum-free CD OptiCHO, and the culture supernatant was collected after a certain period of time. Equilibrate HiTrapMabSelectSuRe 1ml column (GE Healthcare LifeSciences product, Cat.No: 11-0034-93) with PBS solution of pH 7.4 to 10 bed volumes, flow rate is 0.5ml / min; culture supernatant is filtered and loaded with 0.45μm filter membrane , the flow rate is 0.5ml / min. Wash again for 5-10 bed volumes with PBS solution at pH 7.4 at a flow rate of 0.5 ml / min; elute with 100 mM citric acid buffer (pH 3.6) at a flow rate of 0.5 ml / min and collect the eluted peaks.
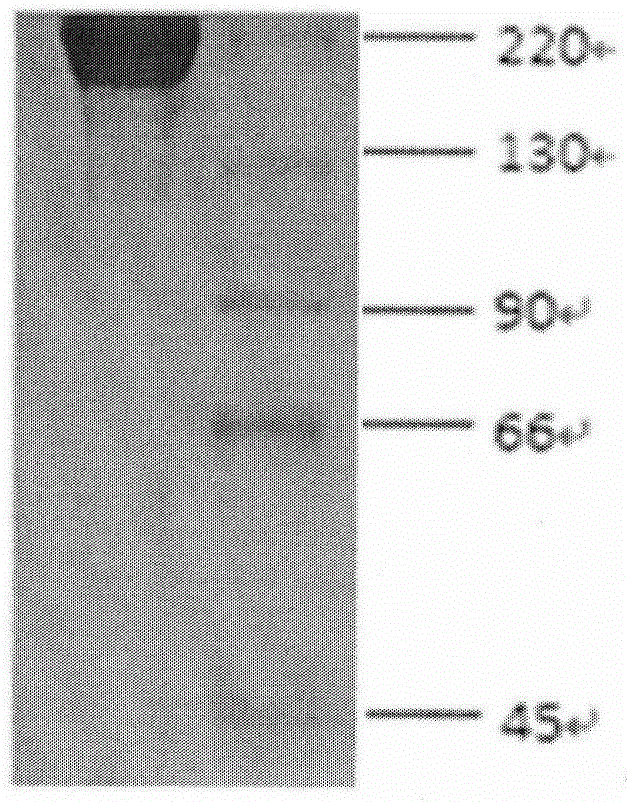
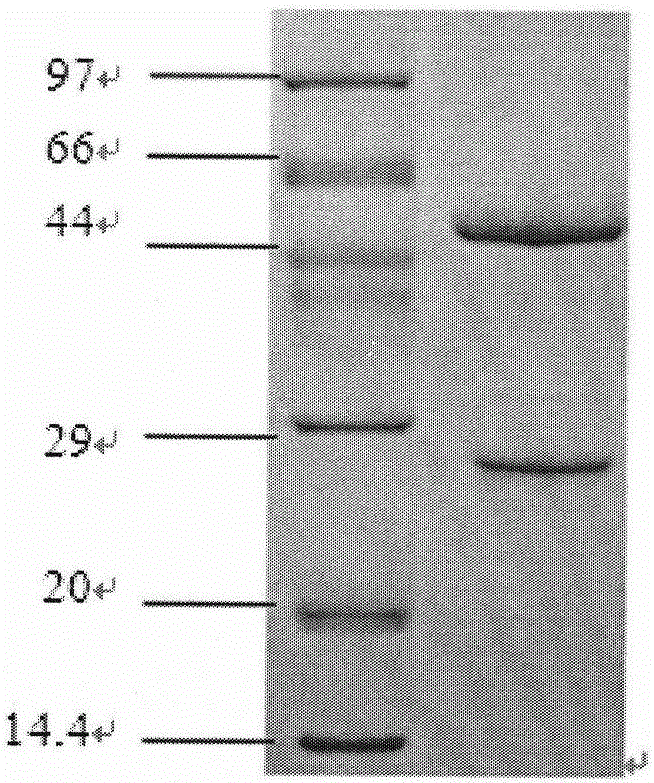
[0052] The expression level of the antibody in the supernatant was detected by ELISA and protein A-HPLC to be 600 mg / L.
[0053] Purified humanized antibody BY01 non-reducing SDS-PAGE electrophoresis and reducing SDS-PAGE electrophoresis see figure 2 and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com