Fluorene derivatives, copolymers and lens using same
A technology of derivatives and copolymers, applied in the field of fluorene derivatives and their copolymers, can solve the problems of poor molding processability, high material cost, practical application obstacles, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
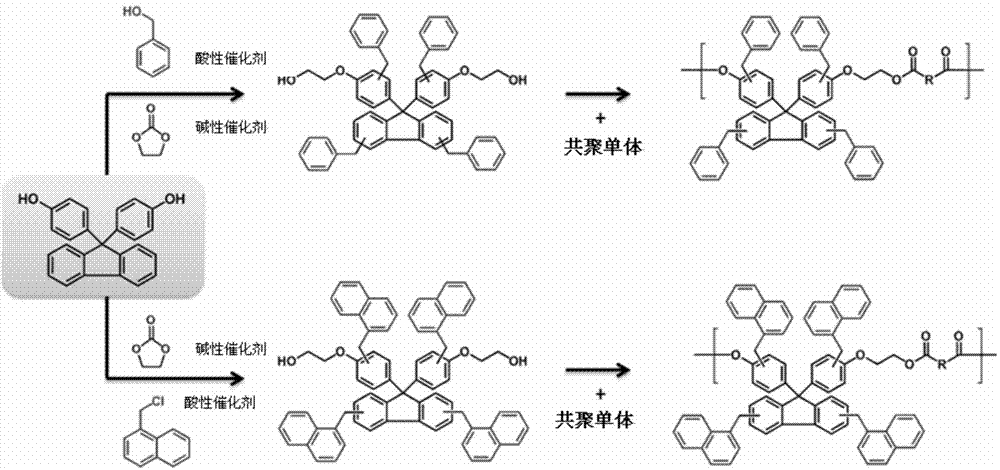
[0107] -2 substituted phenyl fluorene alcohols of chemical formula 4 ( )Synthesis
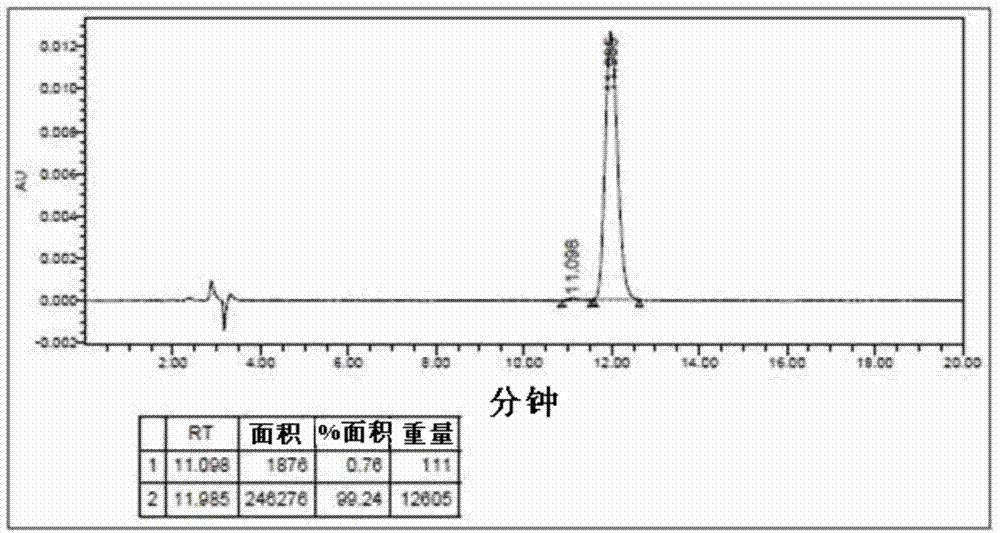
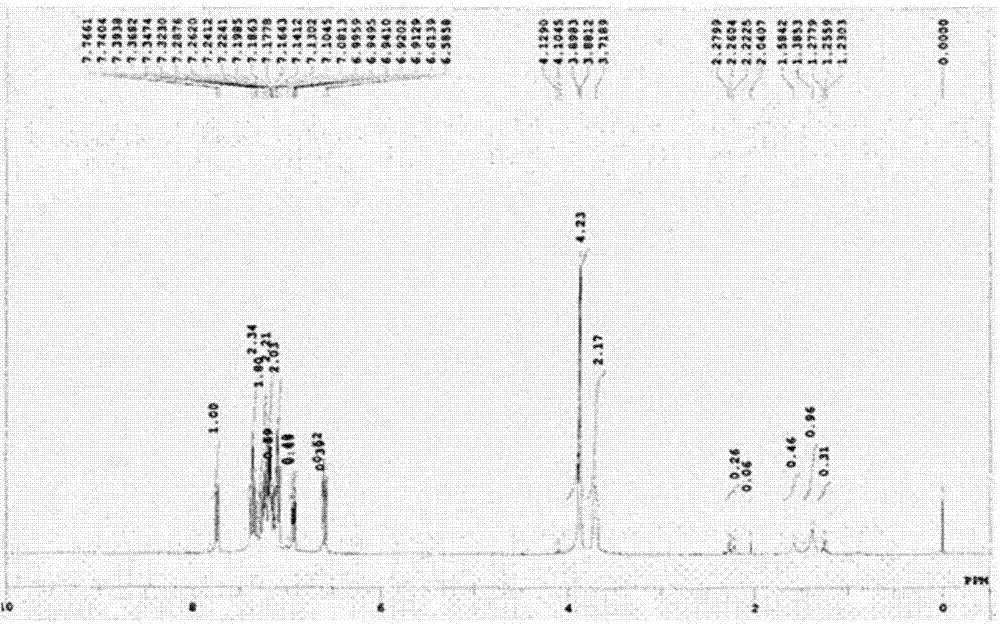
[0108] In a 3L reactor equipped with a reflux device, add 400ml of reaction solvent acetonitrile, then add 9-fluorenone (9-fluorenon), 2-benzylphenol (2-benzylphenol), 3- Mercaptopropionic acid (3-mercaptopropionic acid) was dissolved. The reaction catalyst sulfuric acid was slowly added dropwise there, and the reaction temperature was maintained at 80° C. for 1 hour, and the end point of the reaction was confirmed by TLC. The reactor was cooled and maintained at normal temperature, and an aqueous solution of potassium carbonate was added dropwise to neutralize the reaction liquid to obtain a crystalline crude product. The obtained reaction product was recrystallized using hexane and dichloromethane to obtain a 2-substituted phenylfluorene intermediate with an HPLC purity of 99.2% and a yield of 91%. Take 100g of the above-mentioned 2-substituted phenylfluorene and add it to a 2L 3-port rea...
Embodiment 2
[0111] -Synthesis of 4 substituted phenyl fluorene alcohols of chemical formula 3
[0112] Reaction 6
[0113]
[0114] Carry out the following reaction with reference to the above reaction formula 6.
[0115] Intermediate 1
[0116] 2-(1,5-Dimethyl 2,4-dioxa-3-borabicyclo[3.1.0]hexan-3-yl)-7-(4,4,5,5-tetramethyl- 1,3,2-Dioxaborolin-2-yl)-9-fluorenone){2-(1,5-dimethyl-2,4-dioxa-3-borabicyclo[3.1.0]hexan-3- Synthesis of yl)-7-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-fluoren-9-one)}
[0117] After adding reaction solvent 1,4-dioxin 2500ml in the 12L reactor that reflux device is installed, add 2,7-dibromo-9 fluorenone of 500g, the dipivaloyl diboron of 902g respectively in the reactor ( Bis (pinacolato) diboron), 50g of tetrakis (triphenylphosphine) palladium (Pd (PPh 3 ) 4 ), and 613 g of potassium carbonate were stirred and dissolved, refluxed at 100° C. for more than 30 hours, and the end point of the reaction was confirmed by TLC. Cool the reactor to normal...
Embodiment 3
[0127] Synthesis of Homopolymer Based on -2 Substituted Phenylfluorene
[0128] Dissolve 2-substituted phenylfluorene monomers with a purity of more than 99% in a mixture of sodium hydroxide aqueous solution and dichloromethane, and use phosgene to perform carbonation reaction to obtain a polymer. The GPC molecular weight and Tg are shown in the table 1 is shown. 2-substituted phenyl fluorene alcohols were also prepared by the same synthetic method.
[0129] Table 1
[0130]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com