H7N9 avian influenza illness probability detection kit
A detection kit, avian influenza technology, applied in the determination/inspection of microorganisms, microorganisms, biochemical equipment and methods, etc., can solve problems such as systemic inflammatory response, cytokine storm, and unclear pathogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Sample preparation and sanger sequencing verification
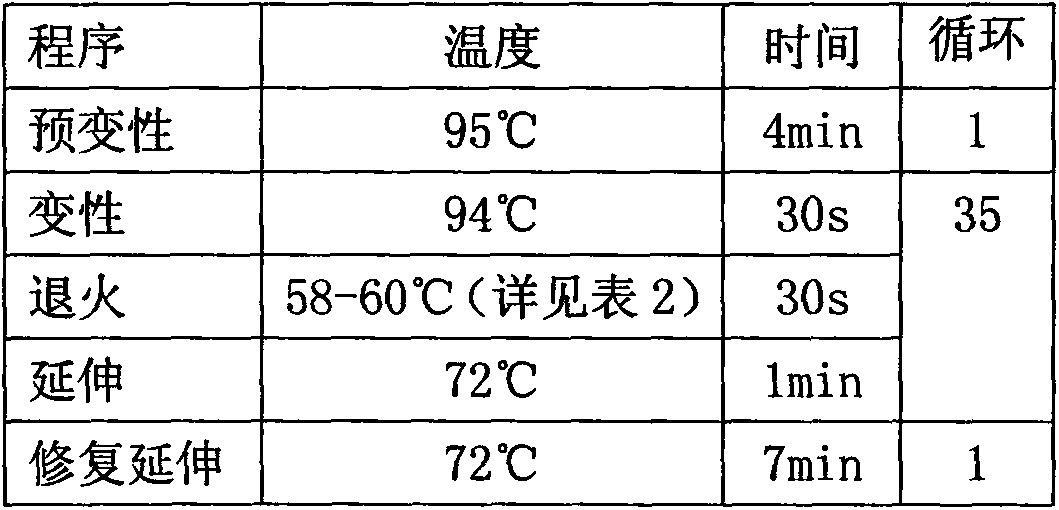
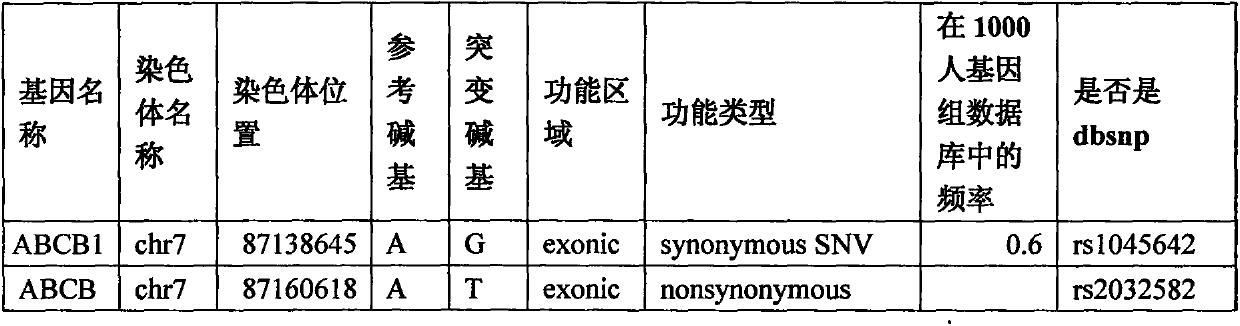
[0027] Detect 5 genes and 10 loci of 8 H7N9 avian influenza patients respectively, divide these 10 loci into 6 fragments (as shown in Table 2), and design primers for the exon sequence of each fragment , Obtain the sequence of each fragment by PCR amplification, product purification, and sanger sequencing, and compare whether it belongs to the mutant type or the wild type according to the sequence determination results, and verify the correlation between each gene and H7N9 avian influenza. The specific method steps are as follows:
[0028] Step 1: DNA Extraction:
[0029] The peripheral blood of 8 patients with H7N9 avian influenza was collected, and the genomic DNA in the peripheral blood leukocytes was extracted using the sQ Blood DNA Kit II produced by omegabiotek (article number: D0714-250), and the concentration of genomic DNA was detected by NanoDrop2000 and agarose gel electrophoresis and purity...
Embodiment 2
[0038] Example 2: H7N9 Avian Influenza Disease Probability Detection Kit and Preparation Method
[0039] 一种H7N9禽流感患病概率检测试剂盒,包含对于CPT2-chr1:53676448&53676401、CPT2-chr1:53679229&53679028、FCGR2A-chr1:161479745、IFITM3-chr11:320772、RPAIN-chr17:5326145和TLR3-chr4:187004074&187004217这 6 pairs of PCR primers for detection of 9 sites in 5 genes.
[0040] The sequences of 6 pairs of PCR primers for detecting these 9 sites are as follows:
[0041]
[0042] According to the method described in step 1 in embodiment 1, extract the DNA of the person to be tested, use the extracted DNA as a template and the above primers to carry out PCR reaction according to the PCR conditions described in step 2 in embodiment 1, and perform PCR according to conventional methods in the art The product was purified, and the purified product was subjected to sanger sequencing. Observe whether the sequence obtained by sequencing has mutations at these 9 sites. If the mutations of these 9 sites are included, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com