Preparation and applications of oral absorption enhancer built based on natural P-glycoprotein inhibitor
A technology of glycoprotein inhibitor and absorption enhancer, which is applied in the field of biomedical materials to achieve the effects of promoting absorption, improving solubility and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
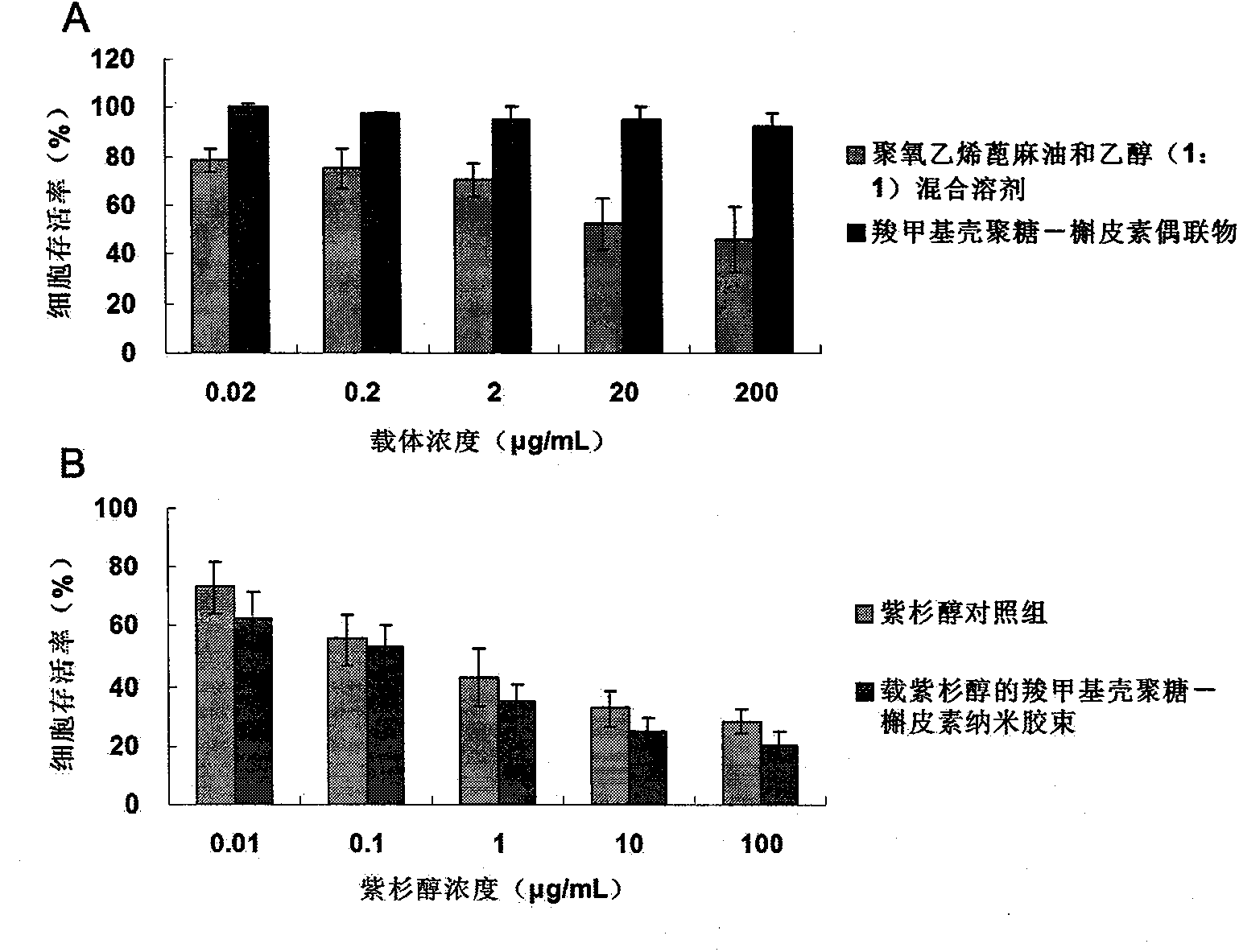
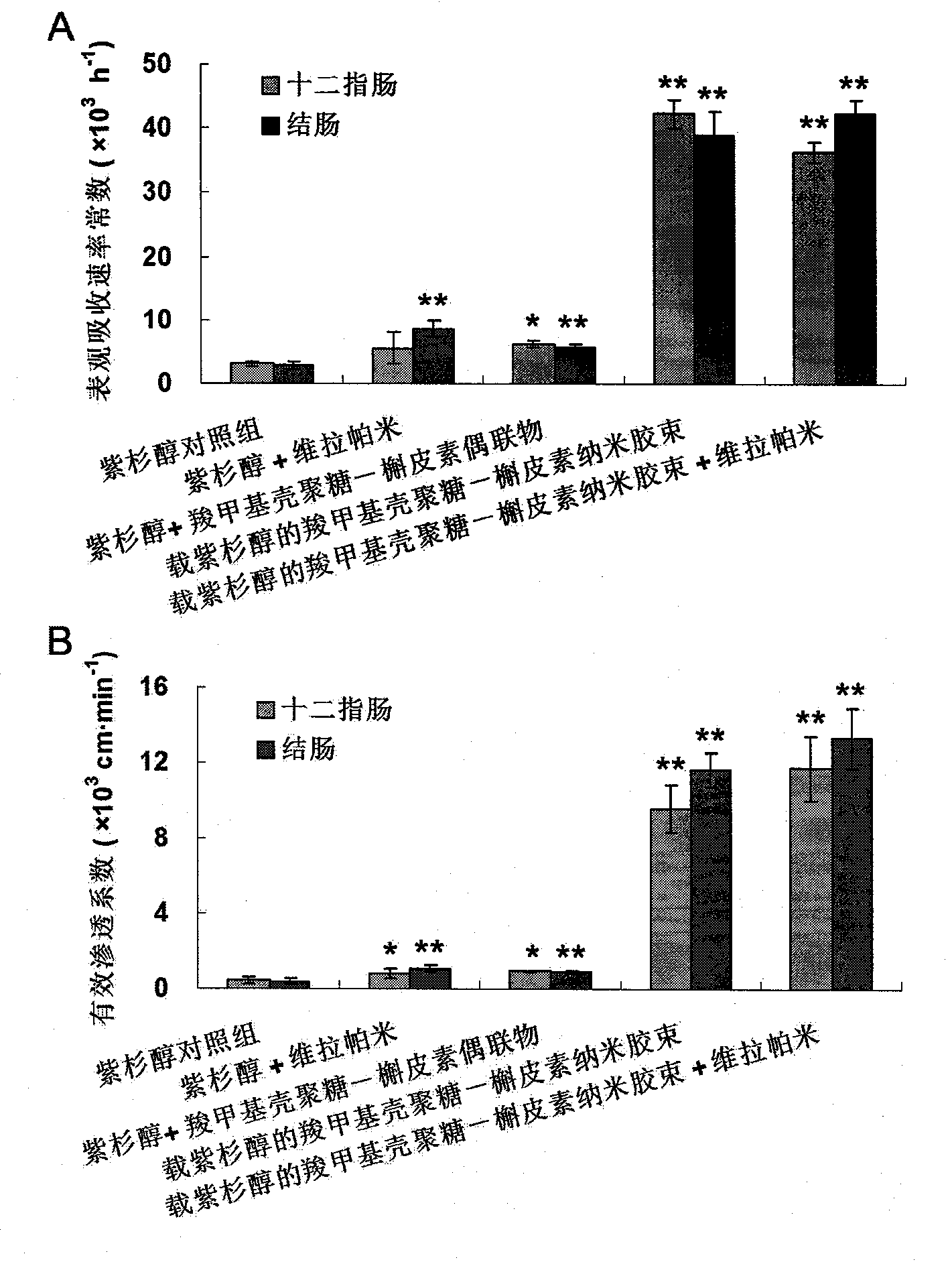
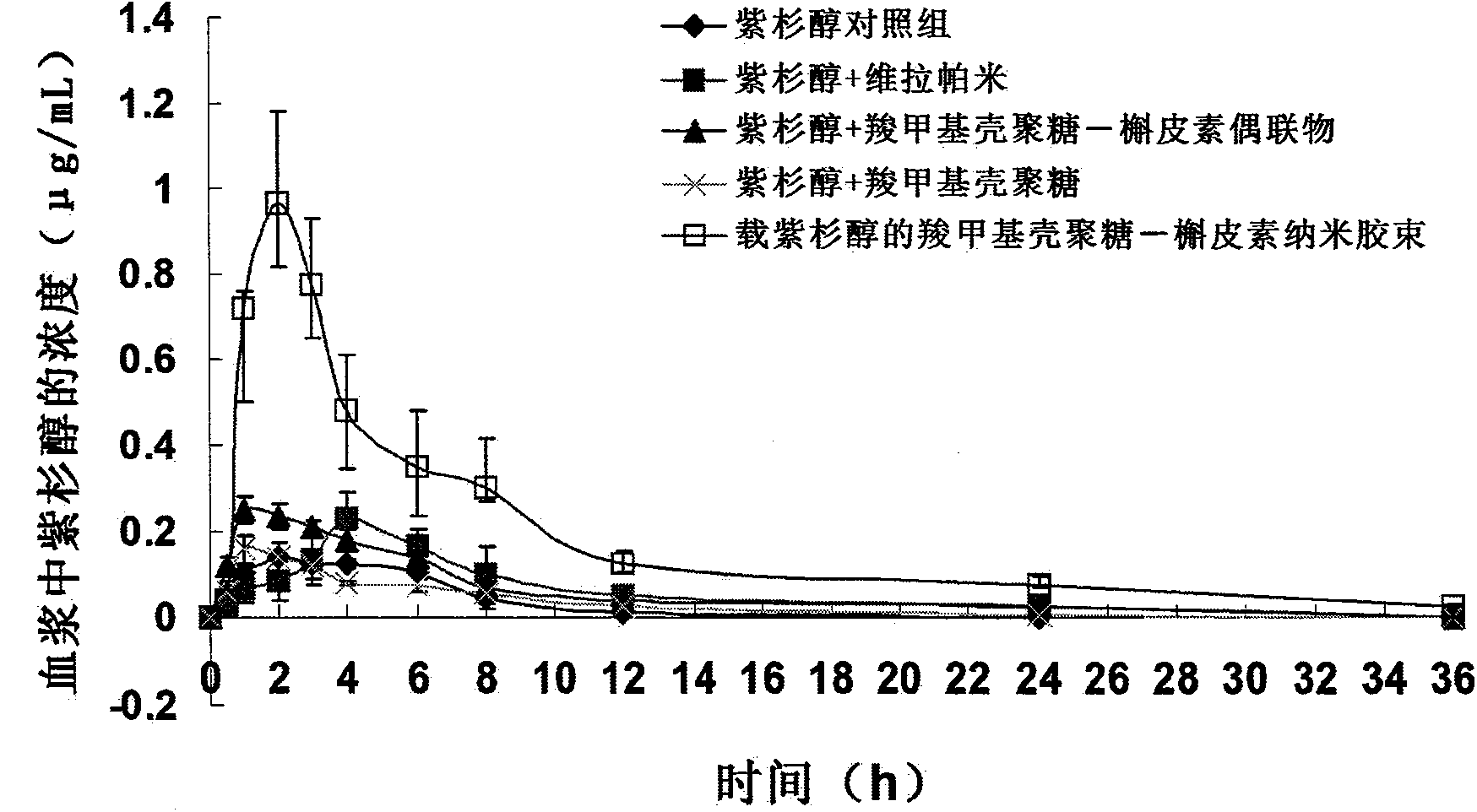
Image
Examples
Embodiment 1
[0048] The synthesis of embodiment 1 carboxymethyl chitosan-quercetin conjugate
[0049] Weigh an appropriate amount of carboxymethyl chitosan and place it in a reaction bottle, add 10 mL of distilled water to swell overnight. Add quercetin in DMF under cold water bath and stirring, then add DMAP (5% of the mass of the reactant), EDC and NHS (EDC:NHS=1:1.4), react at room temperature for 30 minutes, and stir for 24 hours in the dark. After the reaction, the reaction product was precipitated with acetone, and the precipitate was obtained by suction filtration. The precipitate was washed with acetone until it was colorless, and then the reaction product was washed with absolute ethanol until it was colorless. After the solvent was drained, the reaction product was dissolved in water, and the probe was sonicated for 20 minutes in an ice bath, centrifuged at 3000 rpm for 10 minutes, the supernatant was passed through a 0.8 μm filter membrane, and the filtrate was transferred to a ...
Embodiment 2
[0050] The synthesis of embodiment 2 carboxymethyl chitosan-emodin conjugate
[0051] Weigh an appropriate amount of carboxymethyl chitosan and place it in a reaction bottle, add 10 mL of distilled water to swell overnight. Add emodin ethanol solution under cold water bath and stirring, then add DMAP (5% of the reactant mass), EDC and NHS (EDC:NHS=1:2), react at room temperature after 30min, and stir for 24h in the dark. After the reaction, the reaction product was precipitated with acetone, and the precipitate was obtained by suction filtration. The precipitate was washed with acetone until it was colorless, and then the reaction product was washed with absolute ethanol until it was colorless. After the solvent was drained, the reaction product was dissolved in water, and the probe was sonicated for 20 minutes in an ice bath, centrifuged at 3000 rpm for 10 minutes, the supernatant was passed through a 0.8 μm filter membrane, and the filtrate was transferred to a dialysis bag ...
Embodiment 3
[0052] The synthesis of embodiment 3 carboxymethyl chitosan-baicalein conjugates
[0053] Weigh an appropriate amount of carboxymethyl chitosan and place it in a reaction bottle, add 10 mL of distilled water to swell overnight. Add the DMF solution of baicalein in a cold water bath with stirring, then add DMAP (5% of the mass of the reactant), EDC and NHS (EDC:NHS=1:3), react at room temperature for 30 minutes, and stir for 12 hours in the dark. After the reaction, the reaction product was precipitated with acetone, and the precipitate was obtained by suction filtration. The precipitate was washed with acetone until it was colorless, and then the reaction product was washed with absolute ethanol until it was colorless. After the solvent was drained, the reaction product was dissolved in water, and the probe was sonicated for 20 minutes in an ice bath, centrifuged at 3000 rpm for 10 minutes, the supernatant was passed through a 0.8 μm filter membrane, and the filtrate was trans...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com