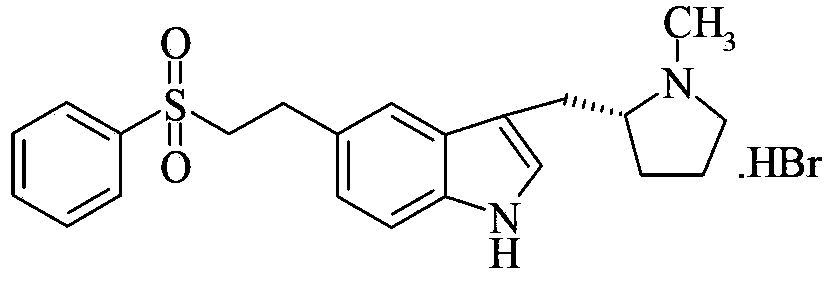
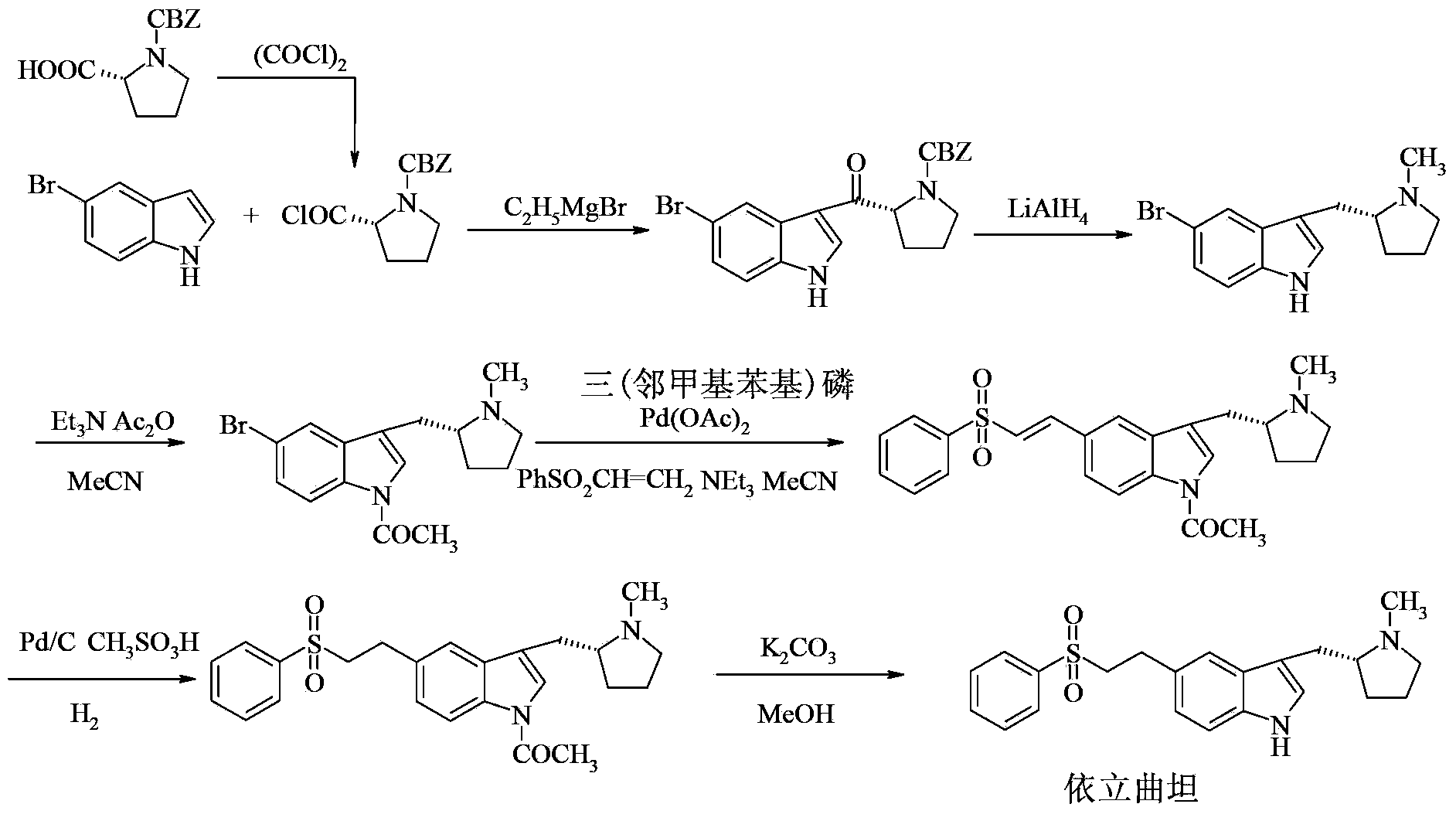
Preparation method for eletriptan intermediate
An intermediate, benzyloxycarbonylpyrrolidine technology, applied in the field of medicinal chemistry, can solve the problems of unstable process, complicated operation, poor selectivity, etc., and achieves the effects of good selectivity, simple operation and reduced dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
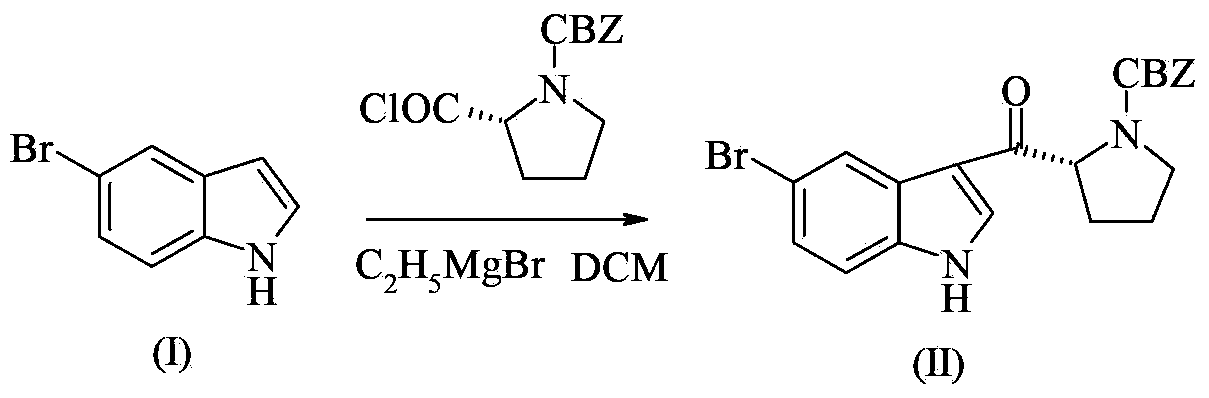
[0030] Add N-benzyloxycarbonyl-D-proline (10.0g, 40.2mmol), 20mL toluene, and 1 drop of DMF to a 100mL four-necked flask in turn, and drop oxalyl chloride (4mL, 46.6mmol) in 5mL of toluene solution at room temperature Put it into a reaction bottle, after the drop, stir at room temperature for 2 hours, concentrate the reaction solution to dryness under reduced pressure, then dissolve the oily product N-benzyloxycarbonyl prolyl chloride in 10 mL of dichloromethane, and set aside.
Embodiment 2
[0032] Add 5-bromoindole (11.8g, 60.3mmol) and 100mL of dichloromethane into a 250ml four-neck flask, cool in an ice bath to 0°C, and slowly add aluminum trichloride solid (9.7g, 72.4mmol) in batches, The internal temperature is controlled at 0-5°C, and the reaction is stirred for 1 hour. Under the condition of 0-5°C, the dichloromethane solution of N-benzyloxycarbonyl prolyl chloride obtained in Example 1 is added dropwise, and the reaction solution is kept at 0-5°C The reaction was stirred for 4h. Under ice bath, slowly drop 60mL of saturated ammonium chloride solution, filter first, and separate layers of the filtrate, wash the organic phase with saturated sodium bicarbonate and saturated brine successively, concentrate under reduced pressure, and wash the crude product with ethyl acetate and n-heptane Mixed solvent (volume ratio: 1:1) was recrystallized and purified, and dried in vacuum at 60°C for 8 hours to obtain the white solid product (R)-3-(N-benzyloxycarbonylpyrroli...
Embodiment 3
[0034] Add 5-bromoindole (11.8g, 60.3mmol) and 100mL of dichloromethane into a 250ml four-neck flask, cool to 0°C in an ice bath, slowly add zinc chloride solid (9.8g, 72.4mmol) in batches, and The temperature is controlled at 0-5°C, and the reaction is stirred for 1 hour. Under the condition of 0-5°C, the dichloromethane solution of N-benzyloxycarbonyl prolyl chloride obtained in Example 1 is added dropwise, and the reaction solution is stirred at 0-5°C. Reaction 4h. Under ice bath, slowly drop 60mL of saturated ammonium chloride solution, filter first, and separate layers of the filtrate, wash the organic phase with saturated sodium bicarbonate and saturated brine successively, concentrate under reduced pressure, and wash the crude product with ethyl acetate and n-heptane Mixed solvent (volume ratio: 1:1) was recrystallized and purified, and dried in vacuum at 60°C for 8 hours to obtain the white solid product (R)-3-(N-benzyloxycarbonylpyrrolidin-2-ylcarbonyl)-5-bromo- 1H-i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com