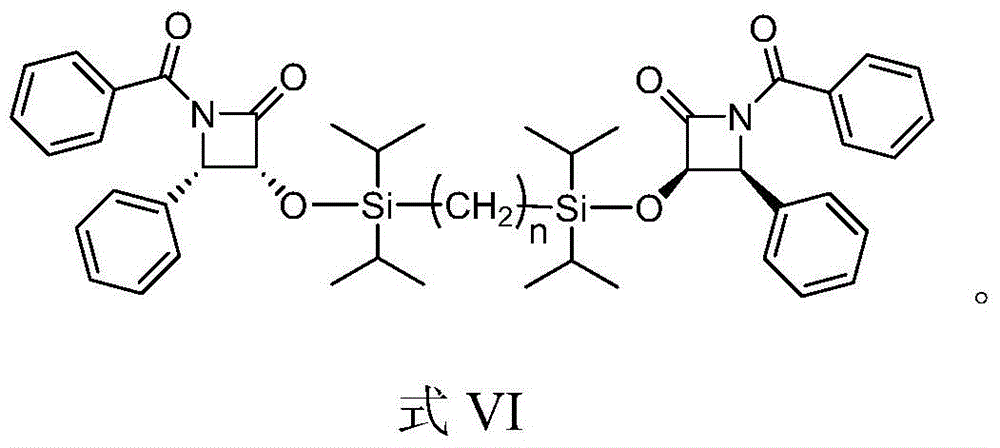
A kind of preparation method of paclitaxel
A paclitaxel and compound technology, applied in the field of drug synthesis, can solve the problems of many by-products, long reaction time, and side reactions, and achieve the effect of less reaction by-products, fast reaction time, and less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
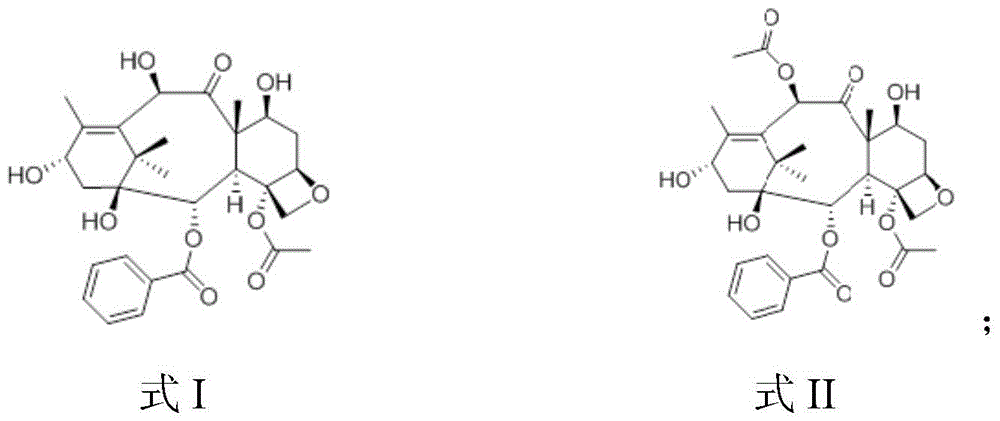
[0055] a, the preparation of baccatin III (i.e. formula II compound)
[0056] At room temperature, add 300g 10-DAB and 30g CeCl3 7H2O into 6L tetrahydrofuran, add 700mL acetic anhydride dropwise under stirring, monitor by TLC, the reaction is complete after 1.5 hours, pour the reaction solution into ice water and let it stand until no white particles precipitate out 305.1 g of the compound of formula II was obtained by suction filtration and drying, and the yield was 94.4% in terms of molar yield; molar yield=(actual production amount of baccatin III / theoretical production amount of baccatin III)×100 %.
[0057] Structural identification of the resulting compound:
[0058] LC-MS(ESI):587.3[M+H] + , Elemental analysis: C 63.46%, H 6.52%, O 30.02%
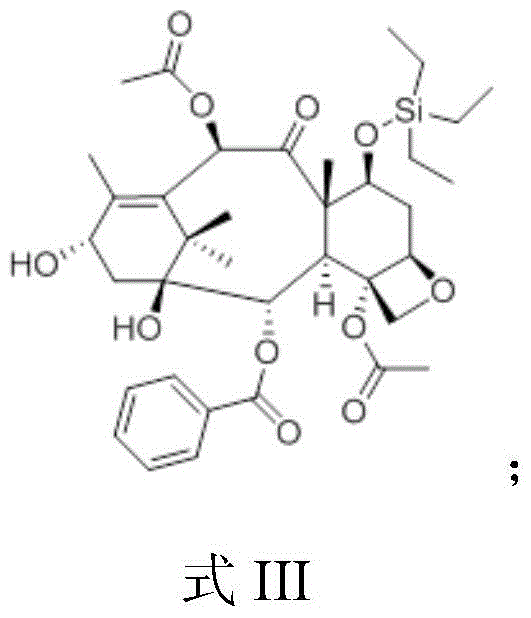
[0059] b, the preparation of 7-TES-baccatin III (i.e. formula III compound)
[0060] At room temperature, dissolve 305.1g of baccatin III in 1200mL of N,N-dimethylformamide, stir and dissolve, add 152.6g of imidazole and 275mL of...
Embodiment 2
[0072] a, the preparation of baccatin III (i.e. formula II compound)
[0073] At room temperature, add 500g 10-DAB and 25g CeCl3 7H2O into 6L tetrahydrofuran, add 1000mL acetic anhydride dropwise under stirring, monitor by TLC, the reaction is complete after 2 hours, pour the reaction solution into ice water and let it stand until no white particles are precipitated 495.5 g of baccatin III was obtained by suction filtration and drying, and the yield was 92.0% in terms of molar yield; molar yield=(actual production amount of baccatin III / theoretical production amount of baccatin III)× 100%.
[0074] b, the preparation of 7-TES-baccatin III (i.e. formula III compound)
[0075] At room temperature, dissolve 495.5g of baccatin III in 1486mL of N,N-dimethylformamide, stir and dissolve, add 148.7g of imidazole and 297.3mL of triethylchlorosilane, monitor by TLC, and the reaction is complete after 3 hours. Add ice water to stop the reaction, extract with dichloromethane, wash the o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com