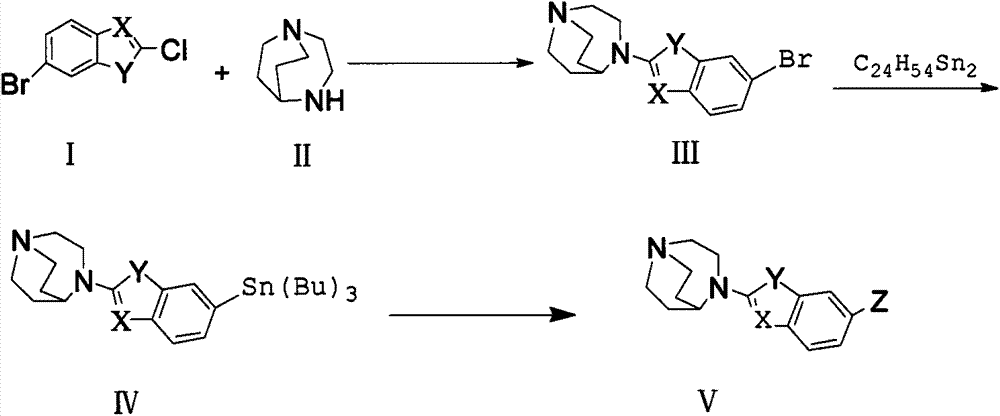
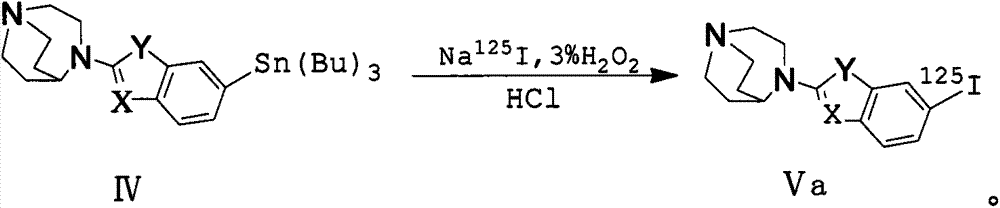
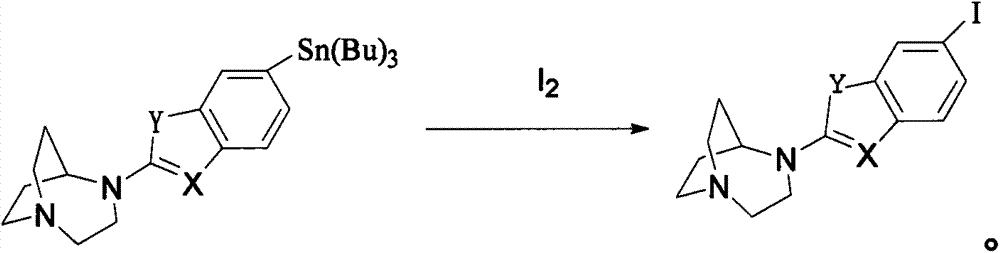
Alpha7 nicotinic acetylcholine receptor ligand and preparation method thereof
A technology for acetylcholine receptors and ligands, which is applied in the field of acetylcholine receptor ligands, can solve the problems of low specific connection, low receptor affinity, etc., and achieve the effect of simple synthesis method and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of compound Va1 and Va3
[0029] 1. 4-bromo-2-fluoroaniline (1.9g, 10mmol), potassium ethyl xanthate (3.53g, 22mmol) were dissolved in 20ml anhydrous DMF, in N 2 Under protection, it was heated to 95° C. and reacted for 4 h (TLC confirmed no reactants). The reaction mixture was cooled to room temperature, diluted with 20ml of water, then 27ml of 1M HCl solution was added to make a precipitate, and stirring was continued for 30min, the solid mixture collected by filtration was rinsed with distilled water, and the solid filter cake was dissolved in 34ml of ethyl acetate, and used without Dry over sodium sulfate, remove ethyl acetate by rotary evaporation, and vacuum-dry the residue to generate 2.24 g of 2-mercapto-5-bromobenzothiazole with a yield of 91%.
[0030]
[0031] ②In an ice-water bath and nitrogen protection, add 20ml of sulfonyl chloride to 2-mercapto-5-bromobenzothiazole (2.46g, 10mmol), react at room temperature for 2h, and p...
Embodiment 2
[0041] Embodiment 2: the preparation of compound Va2 and Va4
[0042] ①Dissolve 2-chlorobenzimidazole (1.52g, 10mmol) in 50ml DMF, then add NBS (N-bromosuccinimide) (1.78g, 10mmol) in 5 times, and react at room temperature for 20h, Add 50ml of water, stir for 18h, filter the precipitate, and wash with ice water repeatedly until it is neutral. Then recrystallized from a solution of methanol:water=1:1 to obtain 0.9 g of 2-chloro-5-bromobenzimidazole with a yield of 40%.
[0043]
[0044] ② Dissolve N,N-diisopropylethylamine (0.7mL, 2.213mmol) and 1,4-diazabicyclo[3.2.2]nonane (0.252g, 2mmol) in 5ml DMF at room temperature, A DMF solution (1 ml) of 2-chloro-5-bromobenzothiazole (0.494 g, 2.012 mmol) was added dropwise to the mixed solution under a steady nitrogen flow, and then the temperature was raised to 110° C. for 12 h. After the reaction was completed, the reaction was cooled to room temperature, extracted with ethyl acetate and water to obtain an organic phase, dried ...
Embodiment 3
[0052] Embodiment 3: the preparation of compound Vb1 and Vb3
[0053] 1. reaction process is with step 1. in embodiment 1, gets as the 2-mercapto-5-bromobenzothiazole in embodiment 1;
[0054] 2. reaction process is with step 2. in embodiment 1, obtains as 2-chloro-5-bromobenzothiazole among the embodiment 1;
[0055] 3. reaction process is with step 3. in embodiment 1, obtains as compound I among the embodiment 1;
[0056] 4. reaction process is with step 4. in embodiment 1, obtains as compound II among the embodiment 1;
[0057] 5. reaction process is with step 5. in embodiment 1, obtains as compound Va3 among the embodiment 1;
[0058] 6. At room temperature, add CuI (0.2285g, 1.2mmol, M=190.45), KF (0.0871g, 1.5mmol, M=58.1), and compound Va3 (385.27mg, 1.0mmol) in Example 1, DMF ( 2ml) was added trimethyl(trifluoromethylsilane) (0.1706g, 1.2mmol, M=142.19), the reaction mixture was stirred under nitrogen protection at 60°C for 24h, the reaction mixture was poured into ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com