Method for quickly and efficiently removing heavy metals in water body
A technology for heavy metals and water bodies, applied in chemical instruments and methods, water pollutants, water/sewage treatment, etc., can solve problems such as poor environmental friendliness, affecting As removal effect, high price, etc., to achieve low price and low cost Expensive, low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
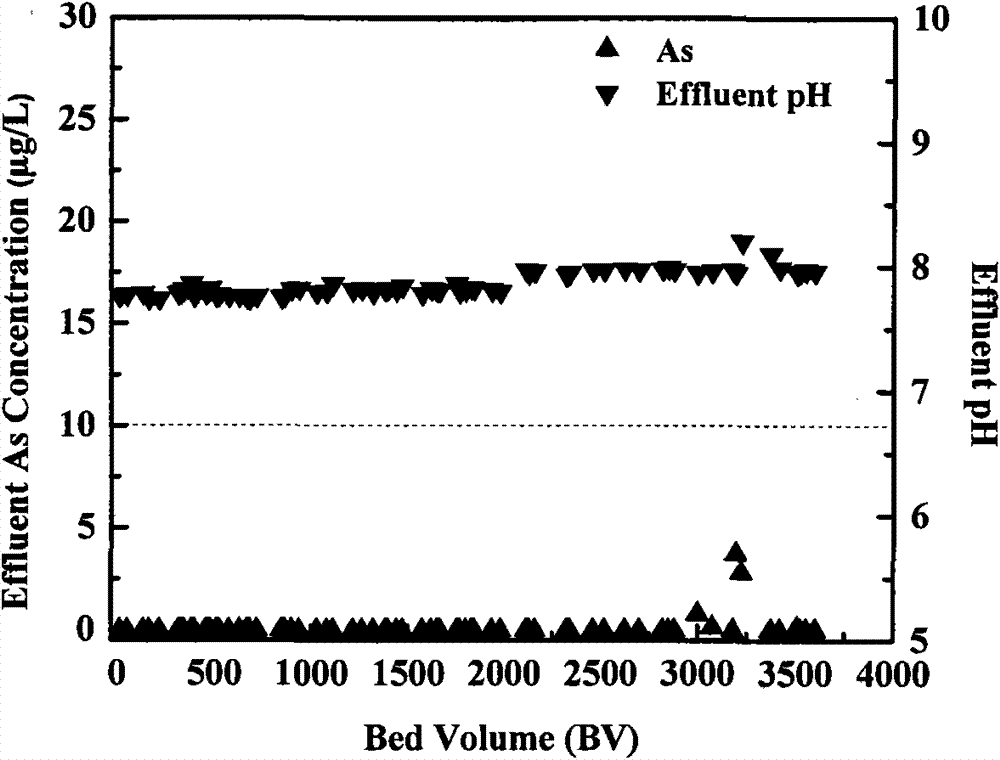
[0025] Embodiment 1: Zero-valent iron / H 2 o 2 Filter column to remove As
[0026] The removal of As(V) was carried out in a 18×400mm plexiglass column. Take 100g of iron sand (particle size 1mm) and fill it in a glass column (the bottom of the column is filled with 1cm glass wool), and then quickly inject 200ml of 10mM H from the top. 2 o 2 Solution, this step is the initialization of the experiment, and then the As(V)-containing raw water is introduced. The As(V)-containing solution in the experiment uses tap water as the raw water background, and sodium asate is added to adjust the As(V) concentration to 200 μg / L , while adding hydrogen peroxide (H 2 o 2 ) adjusted to H in water containing As(V) 2 o 2 The concentration is 0.1mM, the influent water flows in from the top of the adsorption column, about 7.5 empty bed volume (BV) / hour, and the empty bed contact time (HRT) is 8min. Take a certain volume of effluent at regular intervals to measure the concentration of As(V...
Embodiment 2
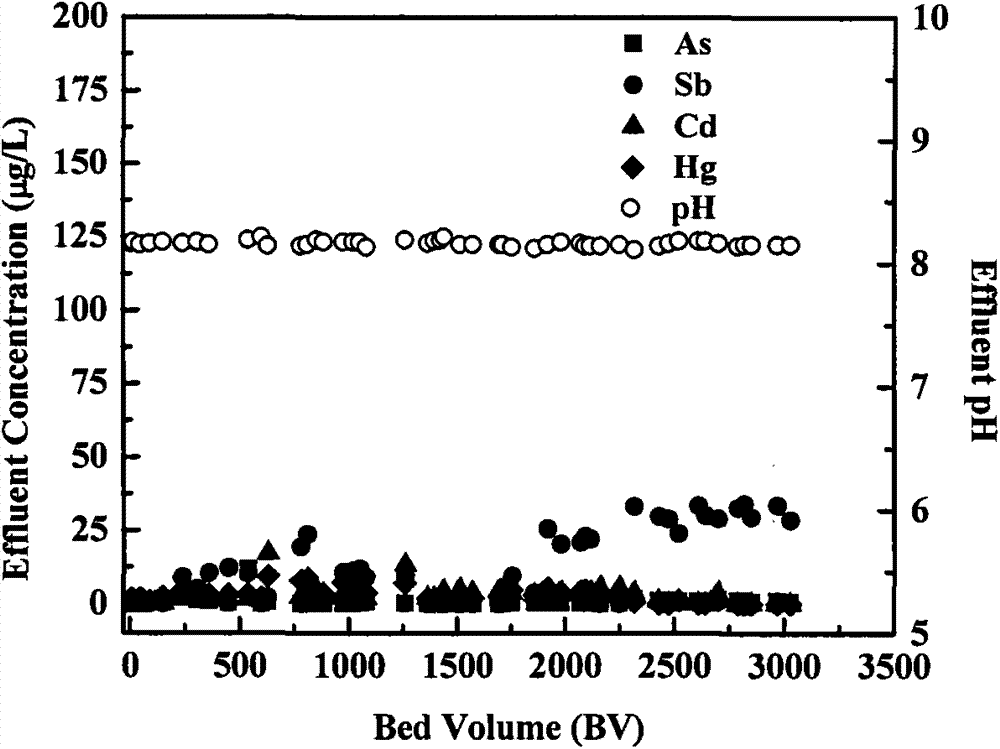
[0028] Example 2: Zero-valent iron / NaClO filter column simultaneously removes As, Sb, Cd, and Hg
[0029] In the heavy metal (As, Sb, Cd, Hg) removal experiment, the heavy metal polluted water body used tap water as the raw water background, and sodium arsenate, potassium pyroantimonate, cadmium chloride, and mercury chloride were added to adjust the concentration to 200 μg / L. The filling and initialization of the reaction column are the same as in Example 1. Pass into the polluted water body that contains heavy metal then, add sodium hypochlorite (NaClO) in the liquid, concentration is 0.5mM, the flow velocity of water is with embodiment 1, gets certain volume effluent liquid at regular intervals, measures four kinds of heavy metals, iron concentration and pH value. Heavy metal removal and effluent pH value such as figure 2 As shown, As and Hg have maintained a very high removal rate, and the removal rate has reached 99-100%. After the outflow volume reaches 3000BV, there ...
Embodiment 3
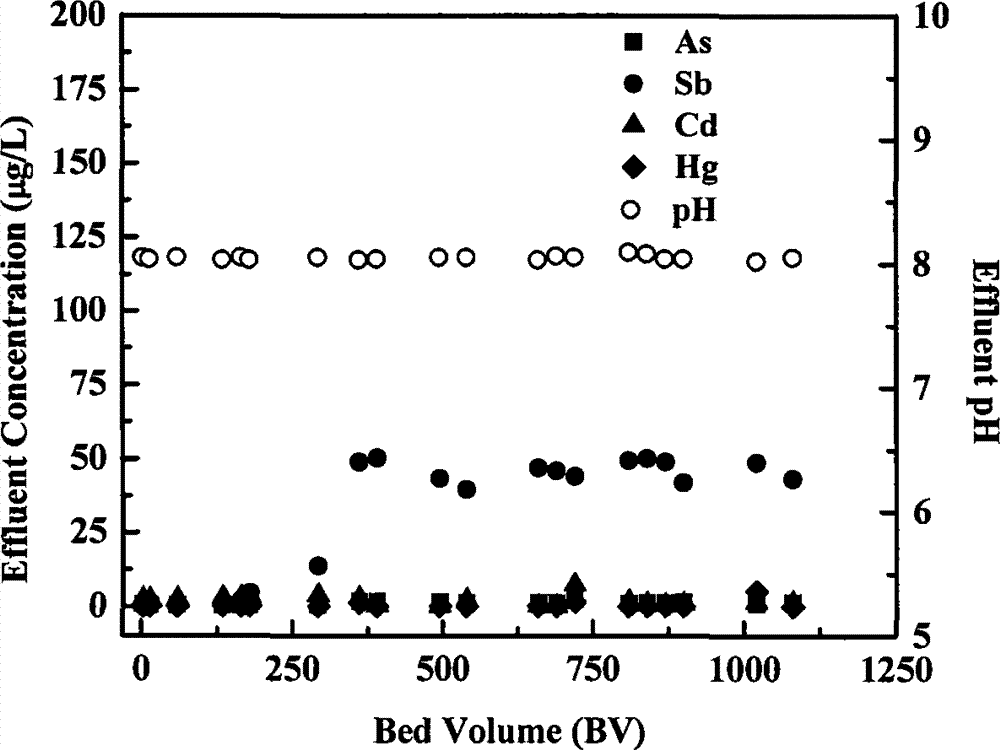
[0030] Embodiment 3: Zero-valent iron / KMnO 4 Filter column removes As, Sb, Cd, Hg at the same time
[0031] The filling, initialization, raw water configuration, and influent flow rate of the filter column are the same as in Example 2, and the influent KMnO 4 The concentration is 0.5mM. The heavy metal removal effect and the pH value of the effluent are as follows image 3 shown. with zero valent iron / H 2 o 2 , Zero-valent iron / NaClO filter system is similar, As, Hg has maintained a very high removal rate, reaching 99-100%, after the outflow volume reaches 1000BV, there is no sign of breakthrough (column experiment is still in progress); Cd The removal effect of Sb also remains good, followed by the removal effect of Sb, which is maintained at about 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com