Preparation method of crystal form of gefitinib Form 1
A technology of gefitinib and crystal form, applied in the field of preparation of gefitinib Form 1 crystal form, can solve the problems of cumbersome operation, poor process stability, poor crystal form purity, etc., and achieve high yield and controllable increase Sexuality and stability, reducing the effect of finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
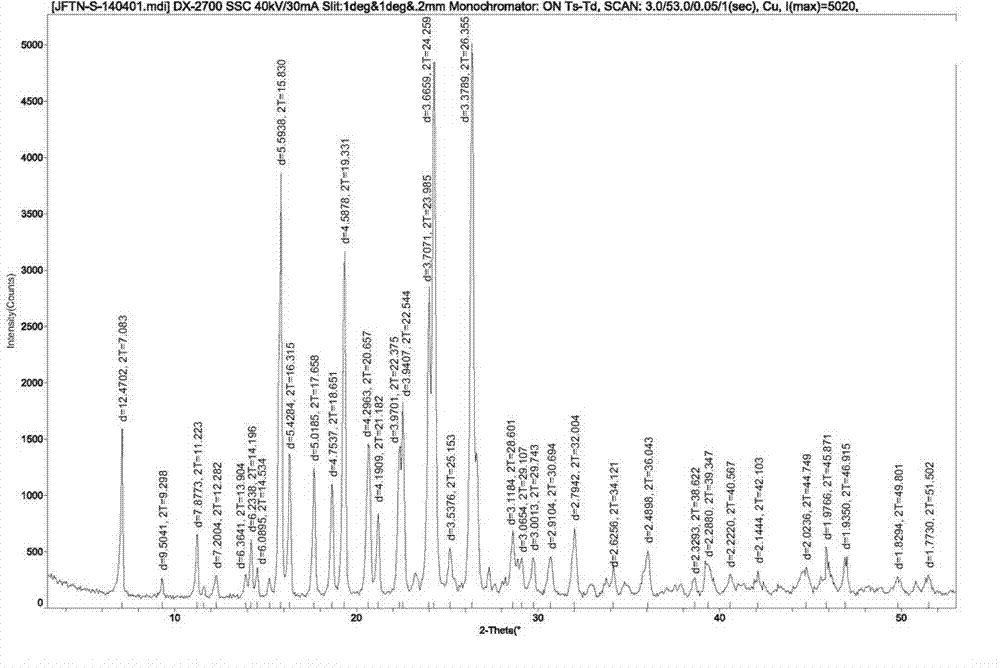
[0025] Embodiment 1 Preparation of gefitinib crystal form I
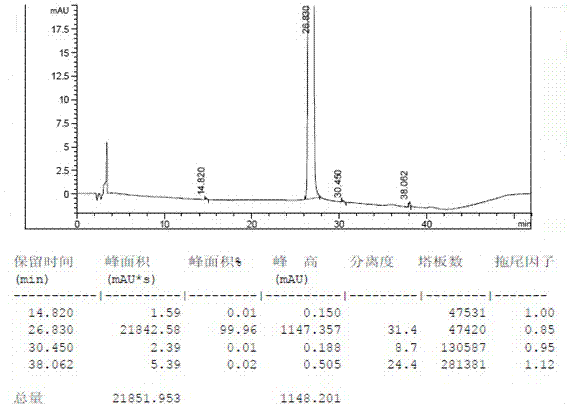
[0026] Add 10.0g of crude gefitinib, 20g of N,N-dimethylformamide and 20g of ethyl acetate into the reaction flask, heat to 60~65°C under stirring, dissolve the solid completely, add activated carbon for decolorization, Filtrate while hot to remove activated carbon, add 40g of purified water dropwise to the mother liquor, naturally cool down to 15~25°C, stir and crystallize for 4 hours, filter, and dry the solid in vacuum (temperature 60~65°C, vacuum degree ≥0.08Mpa) to obtain 9.65g Gefitinib crystal form I, the yield is 96.5%, the chromatographic purity is 99.96%, the maximum single impurity is 0.02%, and the water content (Karl-Fischer method): 0.3%.
Embodiment 2
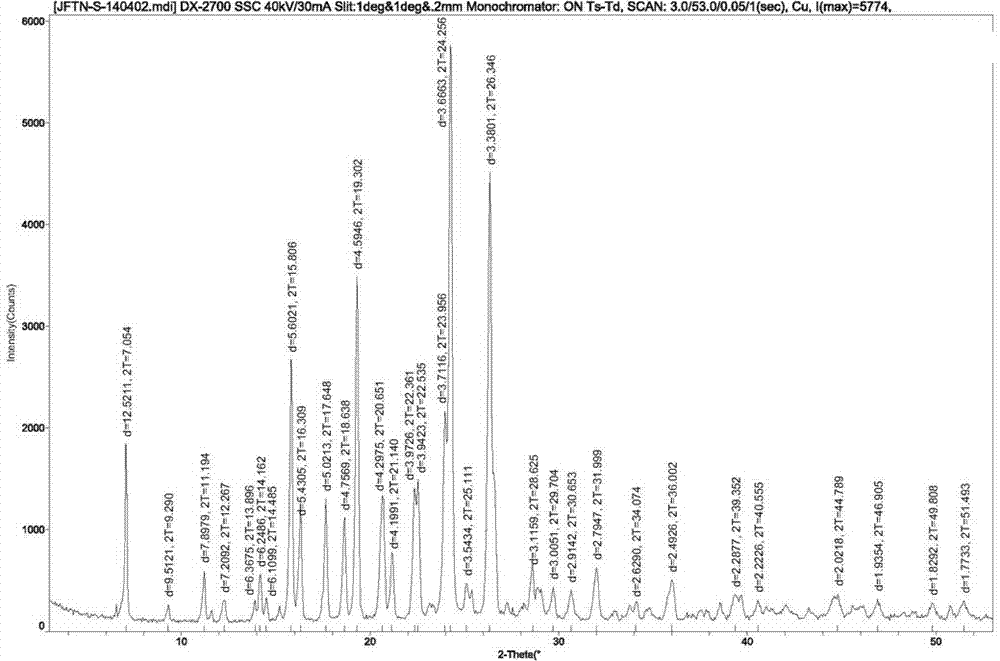
[0027] Embodiment 2 Preparation of gefitinib crystal form I
[0028] Add 10.0g of crude gefitinib, 20g of N,N-dimethylformamide and 20g of acetone in the reaction bottle, heat it to 55~60℃ under stirring to dissolve the solid completely, add activated carbon for decolorization, and Remove the activated carbon by filtration, add 40g of purified water dropwise to the mother liquor, naturally cool down to 15~25°C, stir and crystallize for 4 hours, filter, and dry the solid in vacuum (temperature 60~65°C, vacuum degree ≥ 0.08Mpa) to obtain 9.36g Jifei Tinib crystal form I, yield 93.6%, chromatographic purity 99.92%, maximum unmixed 0.04%, moisture (Karl Fischer method): 0.4%.
Embodiment 3
[0029] Example 3 Preparation of Gefitinib Form I
[0030] Add 10.0g of crude gefitinib, 20g of N,N-dimethylformamide and 20g of acetonitrile in the reaction flask, heat to 60~65°C under stirring to completely dissolve the solid, add activated carbon for decolorization, and heat Remove the activated carbon by filtration, add 40g of purified water dropwise to the mother liquor, naturally cool down to 15~25°C, stir and crystallize for 4 hours, filter, and dry the solid in vacuum (temperature 60~65°C, vacuum degree ≥ 0.08Mpa) to obtain 9.23g Jifei Crystal form I of Tini, the yield is 92.3%, the chromatographic purity is 99.95%, the maximum simplex is 0.03%, the water content (Karl Fischer method): 0.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com