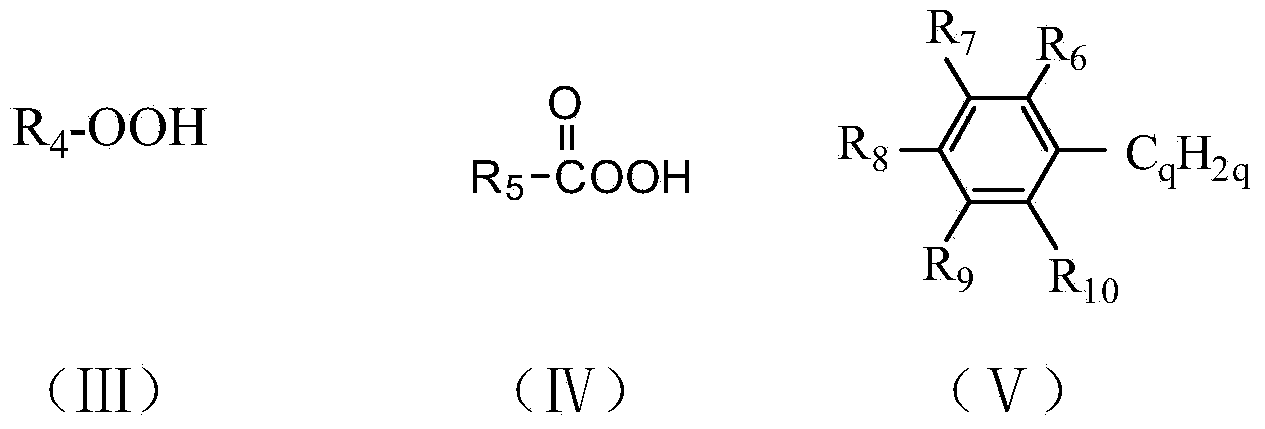Photodissociation reaction method of benzothiophene compound for oxidative desulfurization
A technology of benzothiophene and oxidative desulfurization, which is applied in the petroleum industry, processing hydrocarbon oil, refining hydrocarbon oil, etc., can solve the problem of C-S bonds being difficult to break, and achieve the effect of reducing the loss of hydrocarbons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
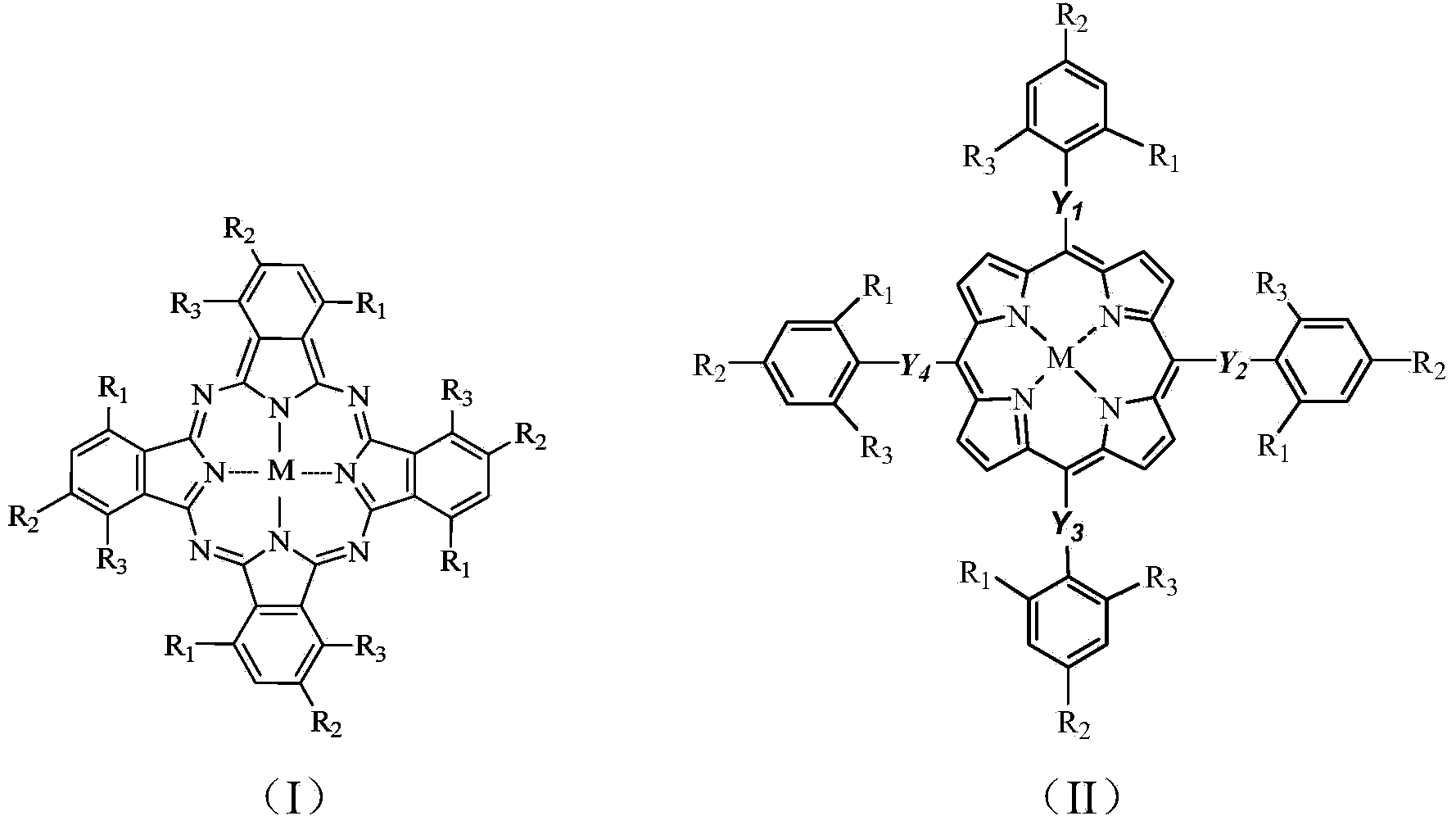
[0040] Weigh 30mg (0.15mmol) dibenzothiophene (DBT), 5mg o-chlorotetraphenylporphyrin iron (that is, M=Fe,R in general formula (II) 1 =Cl,R 2 =R 3 =H,Y 1 =Y 2 =Y 3 =Y 4 =C m , M = 0), dissolved in 30mL methanol solution, slowly drip 54mg (0.9mmol) of tert-butanol peroxide (TBHP) in the reaction mixture, the mixture was stirred under the 250w high pressure mercury lamp for reaction 2.5 Keep the reaction temperature at 36°C with cooling water circulation. After chromatographic column separation and nuclear magnetic resonance detection, the conversion rate of dibenzothiophene C-S bond cleavage in the reaction product was 95.21%.
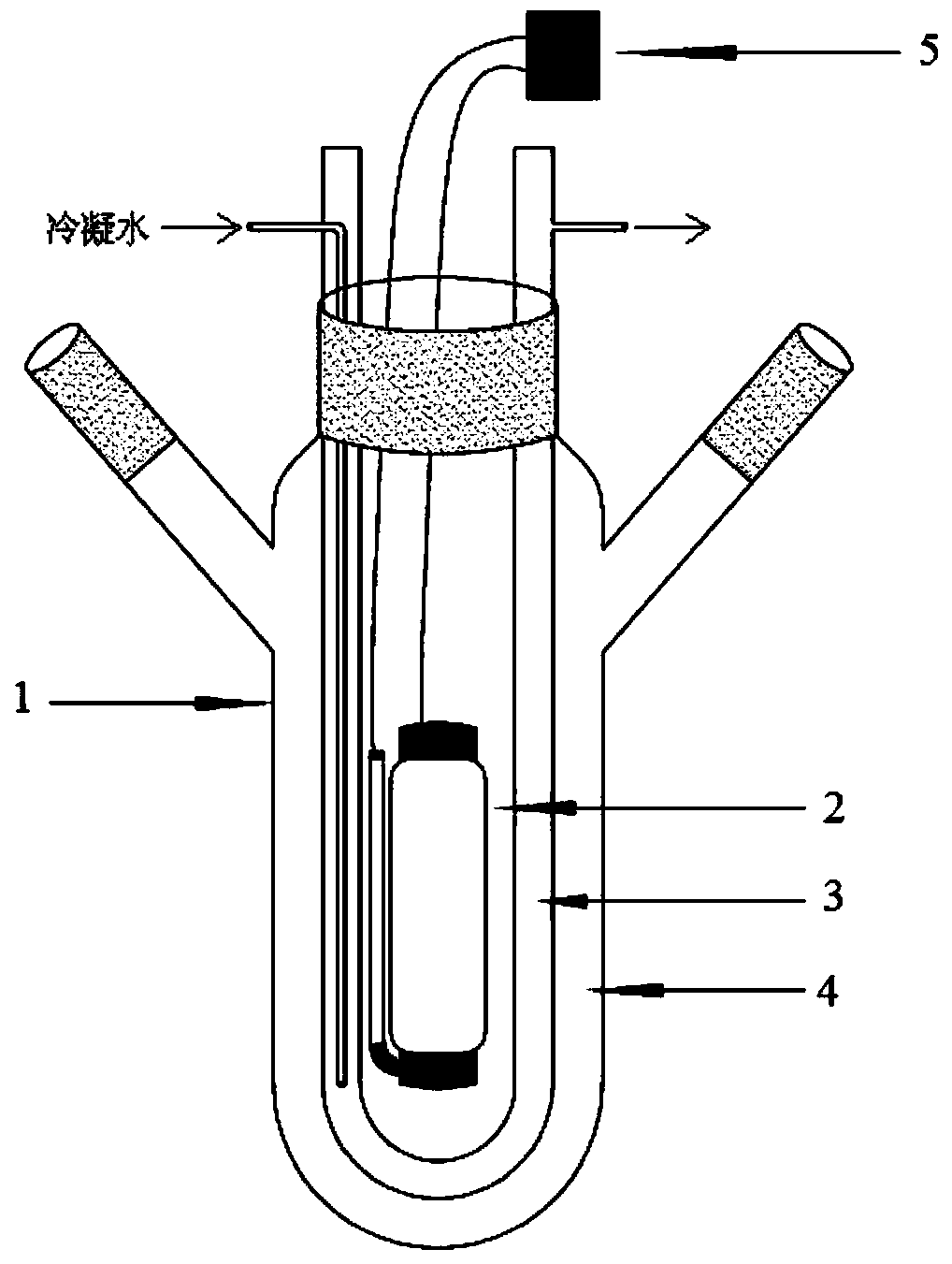
[0041] The above reaction preferably adopts such as figure 1 It is carried out in the reaction device shown, specifically: a three-port reactor 1, a U-shaped condenser tube 3 is installed from the center port, a lamp 2 is arranged in the U-shaped area of the condenser tube, and a light switch 5 controls the on and off of the lamp 2 . In the three-port ...
Embodiment 2
[0044] Weigh 30mg 4,6-dimethyldibenzothiophene (DMDBT), 5mg o-chlorotetraphenylporphyrin iron, dissolve in 30mL methanol solution, slowly add 1.81g H 2 O 2 (30% aqueous solution) In the reaction mixture, the mixture was stirred and reacted for 2.5 hours under the irradiation of a 250w high-pressure mercury lamp, and the reaction temperature was maintained at 36°C with cooling water. Detected by high performance liquid chromatography, 100% conversion of 4,6-dimethyldibenzothiophene was detected. After column separation and NMR detection, the conversion rate of 4,6-dimethyldibenzothiophene CS bond cleavage was 93.27. %.
Embodiment 3
[0046] Weigh 30 mg of dibenzothiophene (DBT), 5 mg of iron phthalocyanine (that is, M=Fe, R in general formula (I) 1 =R 2 =R 3 =H,), dissolved in 30mL methanol solution, slowly dripped 0.72mg TBHP into the reaction mixture, the mixture was stirred and reacted under the irradiation of 250w high pressure mercury lamp for 2.5 hours, and the reaction temperature was kept at 25°C by circulating cooling water. After chromatographic column separation and nuclear magnetic resonance detection, the conversion rate of dibenzothiophene C-S bond cleavage in the reaction product was 67.20%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com