Method for separating and detecting ambrisentan and related substances thereof
A technology related to substances and acetonitrile, applied in the field of drug analysis, can solve the problems affecting the precision and accuracy of quantitative analysis results of samples, high requirements for chromatographic instruments, and reduced signal-to-noise ratio, etc., to achieve accurate and reliable analysis results and stable separation baselines. , the effect of low instrument requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
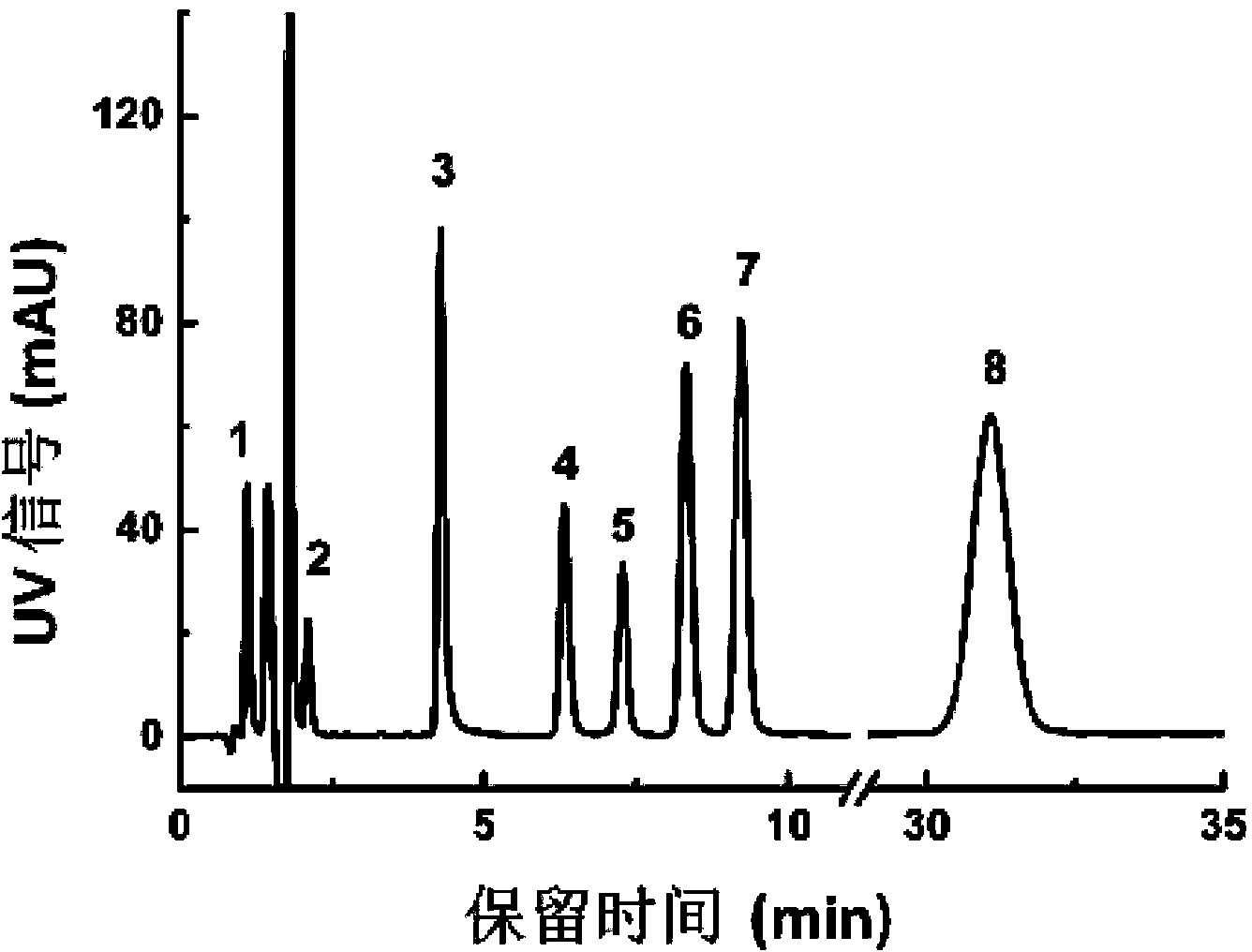
[0021] Containing ambrisentan, benzophenone, 2-hydroxy-3-methoxy-3,3-diphenylpropionic acid (SRS1), (S)-1-(4-nitrophenyl)-ethyl Amine (SRS2), methyl 3,3-diphenyl-2,3-epoxypropionate (SRS3), methyl 2-hydroxy-3-methoxy-3,3-diphenylmethylpropionate (SRS4 ), 4,6-dimethyl-2-methyl-sulfonylpyrimidine (SRS5) and 4,6-dimethyl-2-(2,2-diphenyl-vinyloxy)pyrimidine (DP1) Mixed samples were taken as an example, and an octadecyl-bonded C18 chromatographic column (Waters symmetry, C18, 5 μm, 150mmx4.6mm) was selected, the mobile phase was acetonitrile / water / glacial acetic acid=55 / 45 / 0.25, and the flow rate was 1.0mL / min, column temperature is 35°C, UV detector, detection wavelength is 220nm, see separation results figure 1 . Using this method, the analysis of ambrisentan and its seven related substances can be completed within 35 minutes, the main component peaks are obvious, the column pressure does not change significantly, the separation baseline is stable, and the separation results a...
Embodiment 2
[0023] Taking the determination of the purity of ambrisentan samples as an example, weigh 1.0 mg of ambrisentan solid sample, place it in a 2.0 mL volumetric flask, and add mobile phase to fully dissolve it. An octyl-bonded C8 chromatographic column (Dikma diamonsil, C8, 5 μm, 150mmx4.6mm) was selected, the mobile phase was acetonitrile / water / glacial acetic acid=60 / 40 / 0.25, the flow rate was 0.5mL / min, the column temperature was 30°C, and UV Detector, detection wavelength is 210nm. The method can be used to accurately determine the purity of the ambrisentan sample, the main component peak is obvious, and the trace impurities can be accurately detected.
Embodiment 3
[0025] Taking the determination of the purity of ambrisentan samples as an example, weigh 1.0 mg of ambrisentan solid sample, place it in a 2.0 mL volumetric flask, and add mobile phase to fully dissolve it. An octadecyl-bonded C18 chromatographic column (Waters symmetry, C18, 5 μm, 150mmx4.6mm) was selected, the mobile phase was acetonitrile / water / glacial acetic acid=50 / 50 / 0.2, the flow rate was 0.8mL / min, and the column temperature was 30°C, UV detector, detection wavelength is 190nm. The method can be used to accurately determine the purity of the ambrisentan sample, the main component peak is obvious, and the trace impurities can be accurately detected.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com