Detection method for tetraacetylribose in azacitidine raw material
A detection method, the technology of azacitidine, which is applied in the detection field of impurities, can solve the problems such as unusable detection and inability to detect tetraacetyl ribose, and achieve good peak shape symmetry, simple and easy operation, and high detection efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
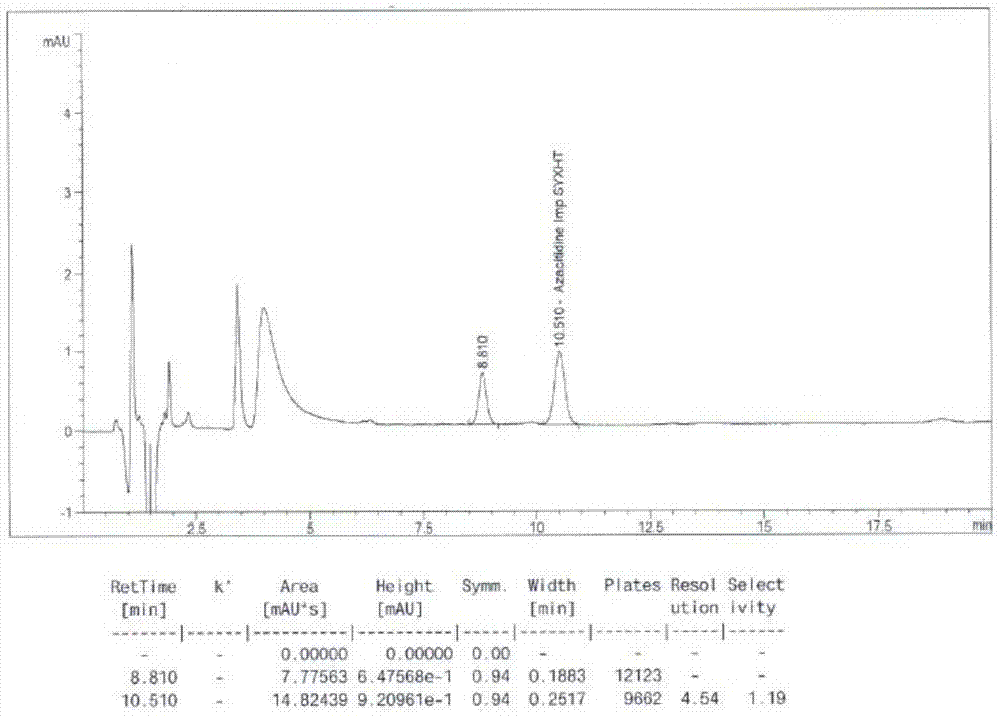
[0027] A detection method for tetraacetyl ribose in azacitidine raw material, adopts high performance liquid chromatography, selects Agilent zorbax SB-C18 chromatographic column, the particle size of the filler is 5 μm, and the specification is 150mm×4.6mm. The volume percentage of water and methanol is 70%: 30% for isocratic elution, the flow rate is 1.0mL / min, the column temperature is 25°C, measure 50μL, 5mg / mL azacitidine aqueous solution for detection, and the detection wavelength It is 210nm, and the elution time is 20min. Test results such as figure 1 shown. Tetraacetylribose impurity peak retention time (R = 10.510min), and adjacent peaks are well separated (resolution R = 4.54), the number of peak theoretical plates is high (N = 9662), and the peak shape has good symmetry (Symm=0.96). It shows that the method has high accuracy and high sensitivity, and can accurately detect the content of the tetraacetyl ribose impurity in the azacitidine raw material.
Embodiment 2
[0029] A detection method for tetraacetylribose in azacitidine raw material, using high performance liquid chromatography, using Agilent XDB-C 18 For the chromatographic column, the particle size of the filler is 5 μm, and the specification is 150mm×4.6mm. The volume percentage of water and methanol is 65%: 35% for isocratic elution, the flow rate is 1.2mL / min, the column temperature is 30°C, and 50μL of 0.5mg / mL azacitidine aqueous solution is measured for detection. The wavelength is 210nm, and the elution time is 20min. Test results: Tetraacetylribose impurity peak retention time (R=8.286min), adjacent peaks are well separated, peak theoretical plate number is high (N=9671), peak shape symmetry is good (Symm=0.98 ). It shows that the method has high accuracy and high sensitivity, and can accurately detect the content of the tetraacetyl ribose impurity in the azacitidine raw material.
Embodiment 3
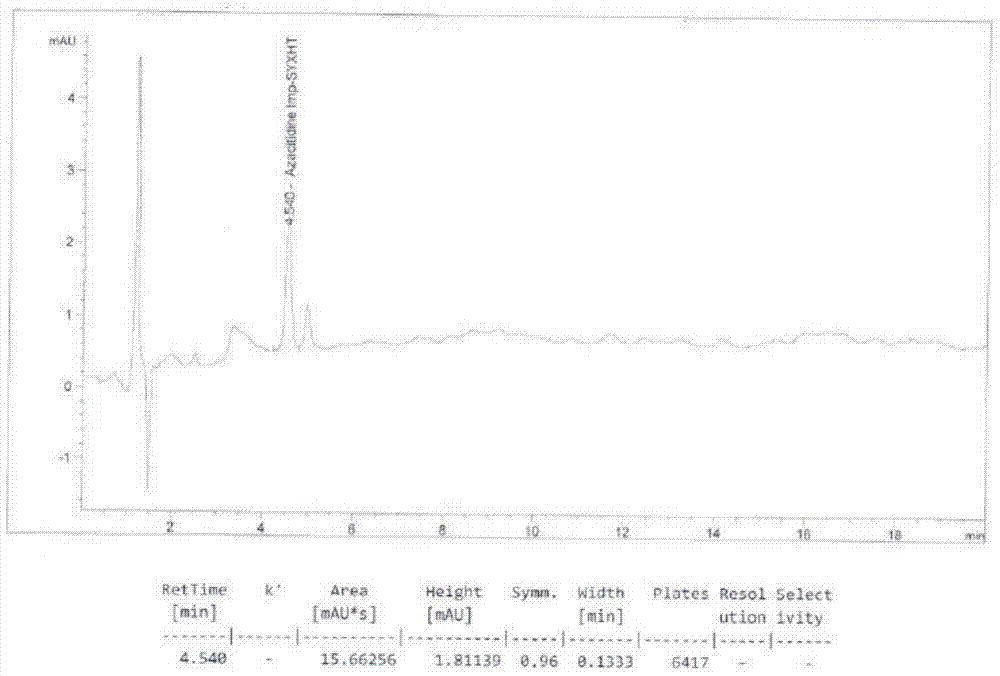
[0031] A detection method for tetraacetylribose in azacitidine raw materials, using high performance liquid chromatography, using Agilent zorbax SB-C18 chromatographic column, the particle size of the filler is 5 μm, and the specification is 150mm×4.6mm. The volume percentage of water and methanol is 55%: 45% for isocratic elution, the flow rate is 0.8mL / min, the column temperature is 35°C, and 50μL of 10mg / mL azacitidine aqueous solution is used for detection. The detection wavelength It is 215nm, and the elution time is 20min. Test results such as figure 2 shown. Tetraacetylribose impurity peak retention time (R=4.540min), and adjacent peaks are well separated, the peak theoretical plate number is high (N=6417), and the peak shape symmetry is good (Symm=0.96). It shows that the method has high accuracy and high sensitivity, and can accurately detect the content of the tetraacetyl ribose impurity in the azacitidine raw material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com