A newborn total galactose detection kit, its use method and preparation method
A detection kit, galactose technology, applied in the field of neonatal galactosemia screening, can solve the problems of poor screening accuracy, high work intensity, and slow detection speed of neonatal galactosemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Neonatal total galactose detection kit
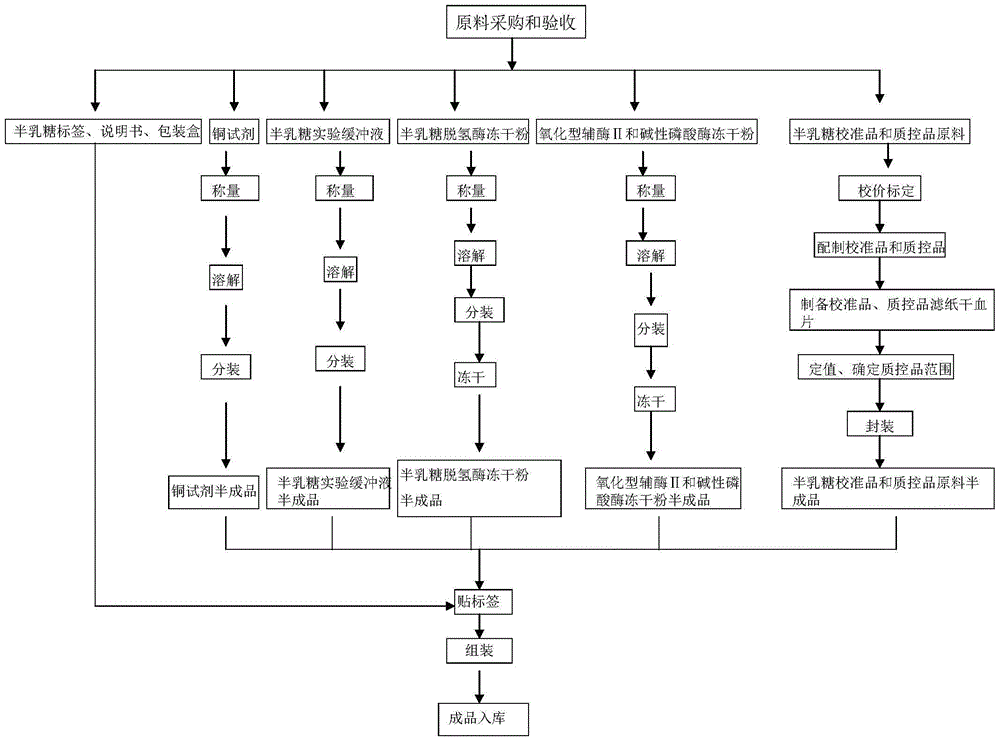
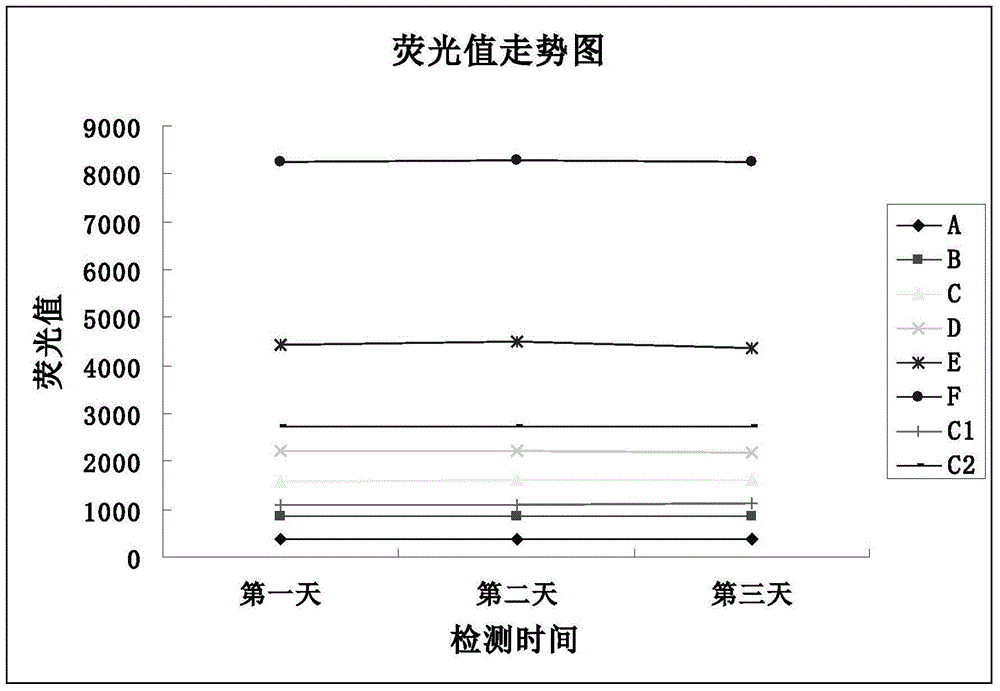
[0059] Neonatal total galactose detection kit contains the following components: 1) Experimental buffer; 2) Mixed lyophilized powder of alkaline phosphatase and oxidized coenzyme II; 3) Lyophilized powder of galactose dehydrogenase; 4) Galactose Calibrator and quality control of filter paper dry blood film; 5) Copper reagent; 6) 96-well microwell blank white reaction plate.
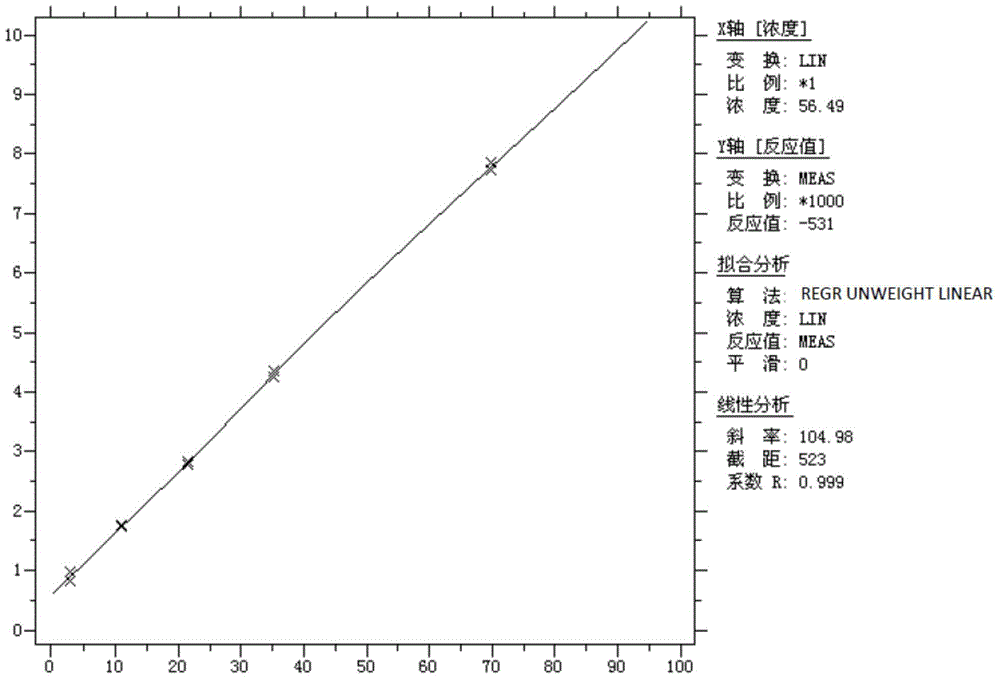
[0060] Wherein, the experimental buffer is a Tris-HCl buffer containing KCl, wherein the molar concentration of Tris-HCl is 10 mmol / L, the molar concentration of KCl is 50 mmol / L, and the pH is 8.0.
[0061]The preparation method of the mixed freeze-dried powder of alkaline phosphatase and oxidized coenzyme II is: a) prepare the mixed solution of alkaline phosphatase and oxidized coenzyme II, which includes alkaline phosphatase with a concentration of 1000U / mL, and the concentration is 46mg / mL oxidized coenzyme II, Tris-HCl with a molar concentration of 1...
Embodiment 2
[0088] Neonatal total galactose detection kit
[0089] Neonatal total galactose detection kit contains the following components: 1) Experimental buffer; 2) Mixed lyophilized powder of alkaline phosphatase and oxidized coenzyme II; 3) Lyophilized powder of galactose dehydrogenase; 4) Galactose Calibrator and quality control of filter paper dry blood film; 5) Copper reagent; 6) 96-well microwell blank white reaction plate.
[0090] Wherein, the experimental buffer is Tris-HCl buffer containing KCl, wherein the molar concentration of Tris-HCl is 10 mmol / L, and the molar concentration of KCl is 10 mmol / L.
[0091] The preparation method of the mixed freeze-dried powder of alkaline phosphatase and oxidized coenzyme II is as follows: a) prepare the mixed solution of alkaline phosphatase and oxidized coenzyme II, wherein the concentration of alkaline phosphatase is 25U / mL, and the concentration of oxidized coenzyme II The concentration of coenzyme II is 1000mg / mL, the molar concen...
Embodiment 3
[0097] Neonatal total galactose detection kit
[0098] Neonatal total galactose detection kit contains the following components: 1) Experimental buffer; 2) Mixed lyophilized powder of alkaline phosphatase and oxidized coenzyme II; 3) Lyophilized powder of galactose dehydrogenase; 4) Galactose Calibrator and quality control of filter paper dry blood film; 5) Copper reagent; 6) 96-well microwell blank white reaction plate.
[0099] Wherein, the experimental buffer is a Tris-HCl buffer containing KCl, wherein the molar concentration of Tris-HCl is 100 mmol / L, and the molar concentration of KCl is 100 mmol / L.
[0100] The preparation method of the mixed freeze-dried powder of alkaline phosphatase and oxidized coenzyme II is as follows: a) prepare the mixed solution of alkaline phosphatase and oxidized coenzyme II, wherein the concentration of alkaline phosphatase is 5000U / mL, and the concentration of oxidized coenzyme II The concentration of coenzyme II is 10mg / mL, the molar co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com