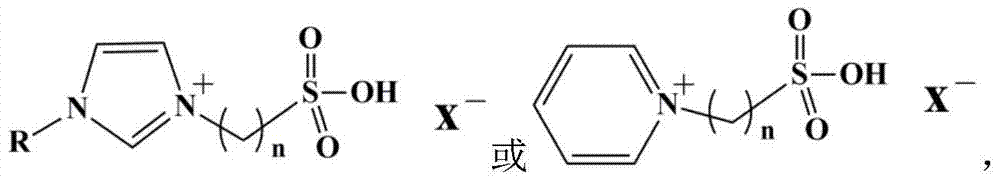
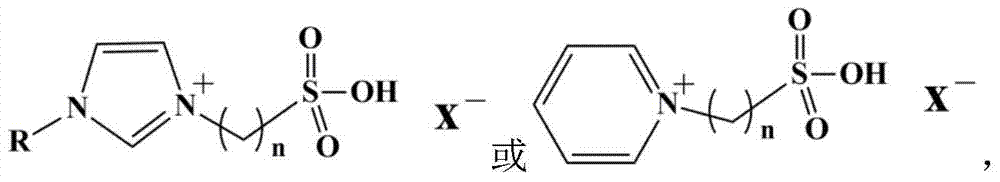
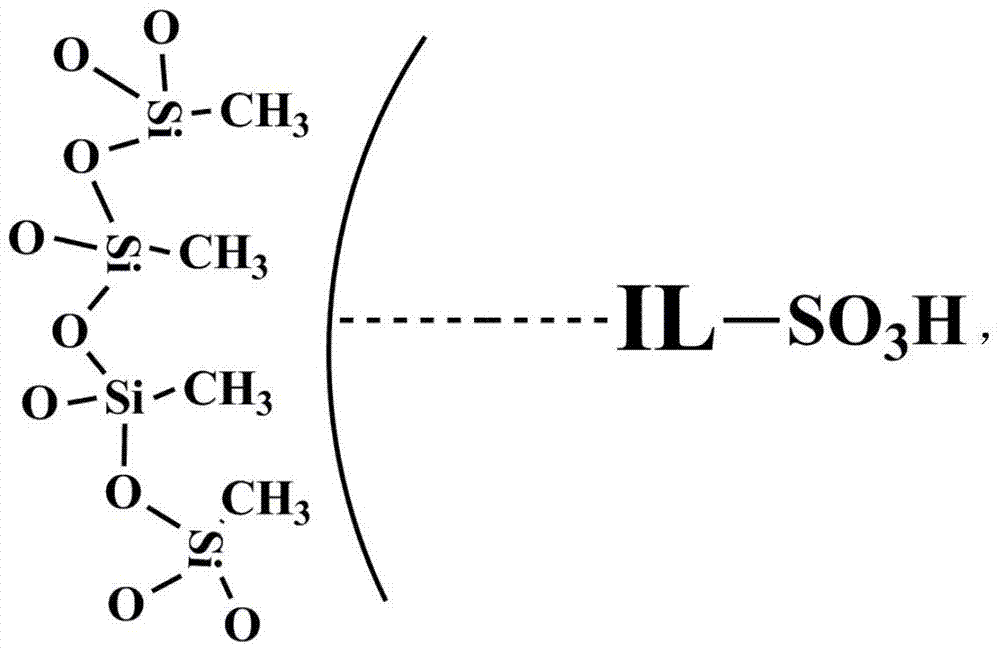Catalyst for conversion of fructose to 5-hydroxymethylfurfural
A technology of hydroxymethyl furfural and a catalyst is applied in the field of converting fructose into 5-hydroxymethyl furfural, and can solve the problems of difficult recovery of catalyst phosphotungstic acid, unfriendly environment, high viscosity of ionic liquid, etc., and achieves a simple and practical loading method, Clarify economic and social benefits, the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a 250mL round bottom flask, take 1mL of an aqueous solution of 0.05mol / L ionic liquid 1-(3-sulfonic acid)-propyl-3-methylimidazolium bisulfate, then add 50mL of liquid paraffin, ultrasonicate for 5min, and then Stand still for 5 minutes and repeat 5 times. Then add 50 mL of 0.3 mol / L methyltrichlorosilane in isooctane solution, stir at room temperature, and feed water vapor (0.1 L / min) at the same time, and react for 5 h. After suction filtration, the obtained solid was dried at 60°C for 24 hours to obtain a catalyst.
Embodiment 2
[0027] In a 250mL round bottom flask, take 1mL of an aqueous solution of 0.04mol / L ionic liquid 1-(3-sulfonic acid)-butyl-3-methylimidazolium bisulfate, then add 50mL of liquid paraffin, ultrasonicate for 5min, and then Stand still for 5 minutes and repeat 5 times. Then add 50 mL of 0.25 mol / L methyltrichlorosilane in isooctane solution, stir at room temperature, and feed water vapor (0.1 L / min) at the same time, and react for 5 h. After suction filtration, the obtained solid was dried at 60°C for 24 hours to obtain a catalyst.
Embodiment 3
[0029] In a 250mL round bottom flask, take 1mL of an aqueous solution of 0.08mol / L ionic liquid 1-(3-sulfonic acid)-propyl-3-methylimidazolium hydrogensulfate, then add 50mL of liquid paraffin, ultrasonicate for 5min, and then Stand still for 5 minutes and repeat 5 times. Then add 50 mL of 0.2 mol / L methyltrichlorosilane in isooctane solution, stir at room temperature, and feed water vapor (0.1 L / min) at the same time, and react for more than 5 hours. After suction filtration, the obtained solid was dried at 60°C for 24 hours to obtain a catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com