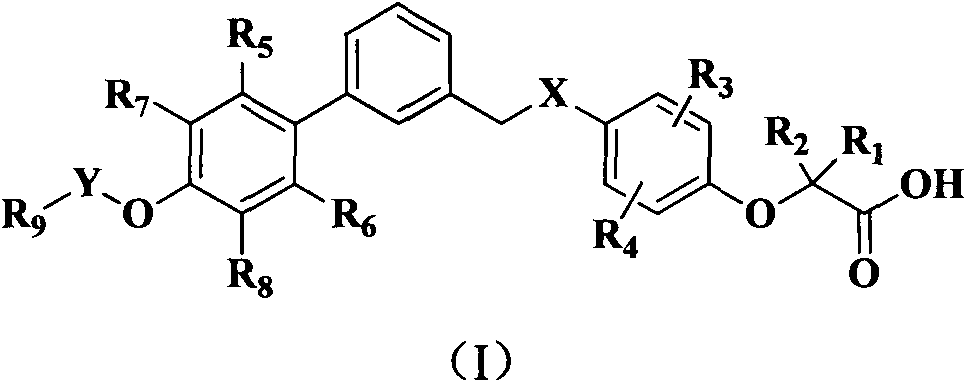
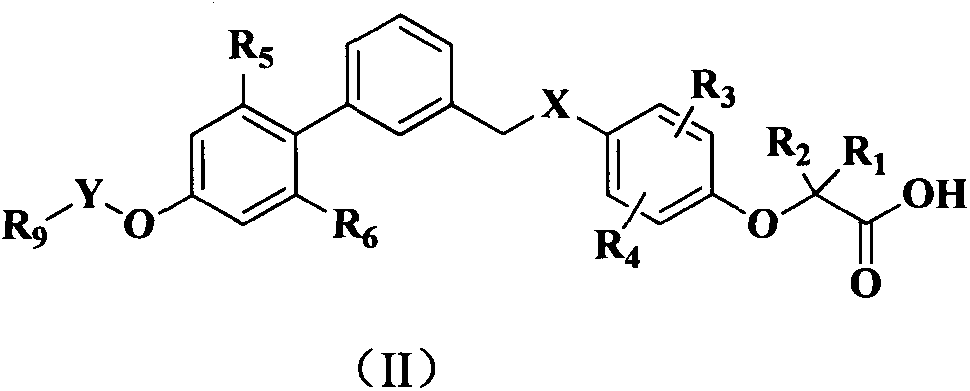
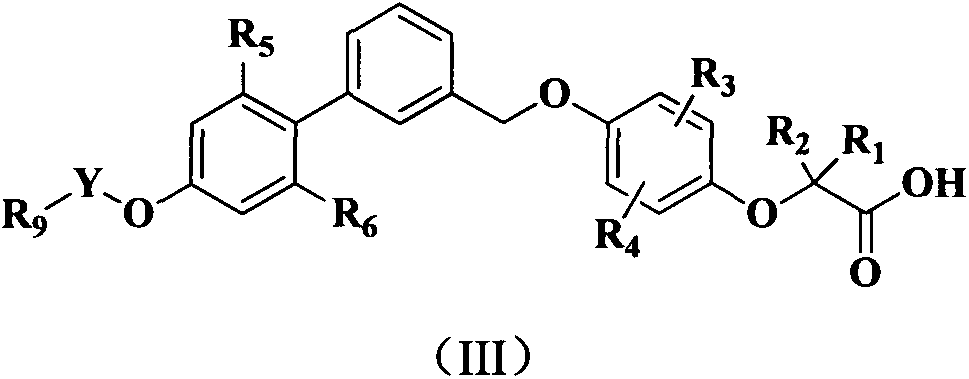
Phenoxyacetic acid derivative, preparation process thereof and use thereof as medicaments
A compound and alkyl technology, applied in the field of phenoxyacetic acid derivatives, their preparation and their use as medicines, can solve problems such as side effects, edema side effects, hypoglycemia and weight gain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] 2-(4-((2′,6′-Dimethyl-4′-(3-(methylsulfonyl)propyl)-[1,1′-biphenyl]-3-)methoxy) Phenoxy)acetic acid (I-1)
[0100]
[0101]
[0102] Compound IV (200mg, 0.49mmol) was dissolved in 10mL acetone, and K was added sequentially 2 CO 3 (134mg, 0.97mmol), catalytic amount of KI and compound V-1 (88mg, 0.49mmol), heated and refluxed overnight, after the reaction, filtered, and the filtrate was concentrated under reduced pressure. The residue was separated by silica gel column chromatography (eluent system: petroleum ether and ethyl acetate) to obtain the target product VI-1 (210 mg, white solid), yield: 84.3%.
[0103] Compound VI-1 (200mg, 0.39mmol) was dissolved in 6mL of tetrahydrofuran, methanol and water (2:3:1) three-component solvent, 2M sodium hydroxide solution (0.78mL, 1.56mmol) was added, and the reaction was stirred at room temperature for 2h After the reaction is over, add 10 mL of water, add 1M hydrochloric acid dropwise to the reaction solution pH 3, extract with eth...
Embodiment 2
[0106] 2-(4-((2′,6′-Dimethyl-4′-(3-(methylsulfonyl)propyl)-[1,1′-biphenyl]-3-)methoxy) 2-methylphenoxy)acetic acid (I-2)
[0107]
[0108] Compound IV (200mg, 0.49mmol) was dissolved in 10mL acetone, and K was added sequentially 2 CO 3 (134 mg, 0.97 mmol), catalytic amount of KI and compound V-2 (95 mg, 0.49 mmol), heated and refluxed overnight, after the reaction was completed, filtered, and the filtrate was concentrated under reduced pressure. The residue was separated by silica gel column chromatography (eluent system: petroleum ether and ethyl acetate) to obtain the target product VI-2 (180 mg, white solid), yield: 70.3%.
[0109] Compound VI-2 (170mg, 0.32mmol) was dissolved in 6mL of tetrahydrofuran, methanol and water (2:3:1) three-component solvent, 2M sodium hydroxide solution (0.64mL, 1.28mmol) was added, and the reaction was stirred at room temperature for 2h After the reaction is over, add 10 mL of water, add 1M hydrochloric acid dropwise to the reaction solution pH 3,...
Embodiment 3
[0112] 2-(2-Chloro-4-((2′,6′-dimethyl-4′-(3-(methylsulfonyl)propyl)-[1,1′-biphenyl]-3- )Methoxy)phenoxy)acetic acid (I-3)
[0113]
[0114] Compound IV (200mg, 0.49mmol) was dissolved in 10mL acetone, and K was added sequentially 2 CO 3 (134mg, 0.97mmol), catalytic amount of KI and compound V-3 (105mg, 0.49mmol), heated and refluxed overnight, after the reaction was over, filtered, and the filtrate was concentrated under reduced pressure. The residue was separated by silica gel column chromatography (eluent system: petroleum ether and ethyl acetate) to obtain the target product VI-3 (220 mg, white solid), yield: 82.7%.
[0115] Compound VI-3 (210mg, 0.40mmol) was dissolved in 6mL tetrahydrofuran, methanol and water (2:3:1) three-component solvent, 2M sodium hydroxide solution (0.79mL, 1.58mmol) was added, and the reaction was stirred at room temperature for 2h After the reaction is over, add 10 mL of water, add 1M hydrochloric acid dropwise to the reaction solution pH 3, extract w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com