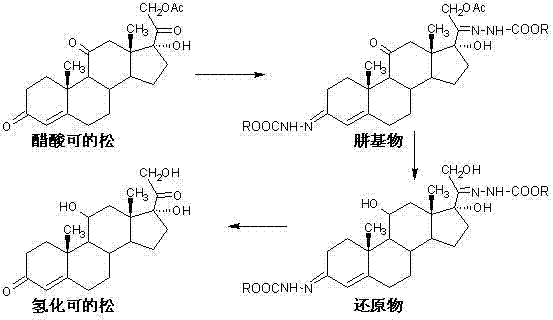
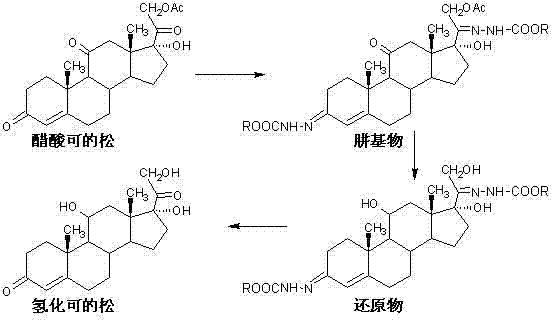
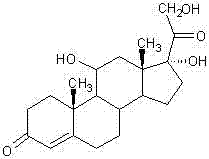
Preparation method of hydrocortisone
A hydrocortisone and ketone-based technology, which is applied in the direction of organic chemistry and steroids, can solve the problems of polluting the site environment, restricting applications, and damaging the health of operators, so as to reduce production costs, increase process yields, The effect of shortening the generation cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 120ml of glacial acetic acid, 280ml of water and 30g of cortisone acetate to the reaction flask in turn, and stir for 10 minutes. Add 18 g of methyl carbazate, and stir and react at 25°C to 30°C for 5 hours. TLC showed that the reaction was complete, and the temperature was lowered to 0°C to 5°C and stirred for 0.5 hours, allowed to stand for more than 2 hours, filtered, washed with water until neutral and dried to obtain 38.6g of methylhydrazine base.
[0027] Add 300ml of methanol, 0.4g of sodium hydroxide dissolved in 60ml of water, and 20g of methylhydrazine to the reaction flask in turn, and stir for 10 minutes. Cool down, and add 5 g of sodium borohydride five times at 10° C. to 15° C., with an interval of 30 minutes each time. After the addition was completed, the reaction was continued at 10°C to 15°C for 8 hours. TLC showed that the reaction was complete, lower the temperature to below 5°C, add dropwise glacial acetic acid to adjust the pH value to 6.5-7....
Embodiment 2
[0030] Add 120ml of glacial acetic acid, 280ml of water and 30g of cortisone acetate to the reaction flask in turn, and stir for 10 minutes. Add 19.5 g of ethyl carbazate, and stir and react at 25°C to 30°C for 6 hours. TLC showed that the reaction was complete, and the temperature was lowered to 0°C to 5°C and stirred for 0.5 hours, allowed to stand for more than 2 hours, filtered, washed with water until neutral and dried to obtain 40.6 g of ethylhydrazine.
[0031] Add 300ml of methanol, 0.4g of sodium hydroxide dissolved in 60ml of water, and 20g of ethylhydrazine to the reaction flask in turn, and stir for 10 minutes. Cool down, and add 6 g of potassium borohydride five times at 10°C to 15°C, with an interval of 30 minutes each time. After the addition was complete, the reaction was continued at 10°C to 15°C for 9 hours. TLC showed that the reaction was complete, lower the temperature to below 5°C, add dropwise glacial acetic acid to adjust the pH value to 6.5-7.0, conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com