A method for extracting high-purity cobra neurotoxin and a pharmaceutical composition containing the toxin
An extraction method and high-purity technology, which can be used in preparations for rectal administration, oral absorption, and oral administration, can solve the problems of poor compliance and slow onset of action, and achieve short onset time, significant analgesic effect, and analgesia. Long-lasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] 1) Take 8 grams of crude cobra venom (purchased from Yujiang Cobra Farm in Jiangxi) and dissolve it in 80 ml of NaAC-HAC solution with a pH of 5.050 mmol / L. After fully dissolving, centrifuge at low temperature (temperature: 0°C--10°C, speed: 3600 rpm, time: 30 minutes), take the supernatant, and wait for the column;
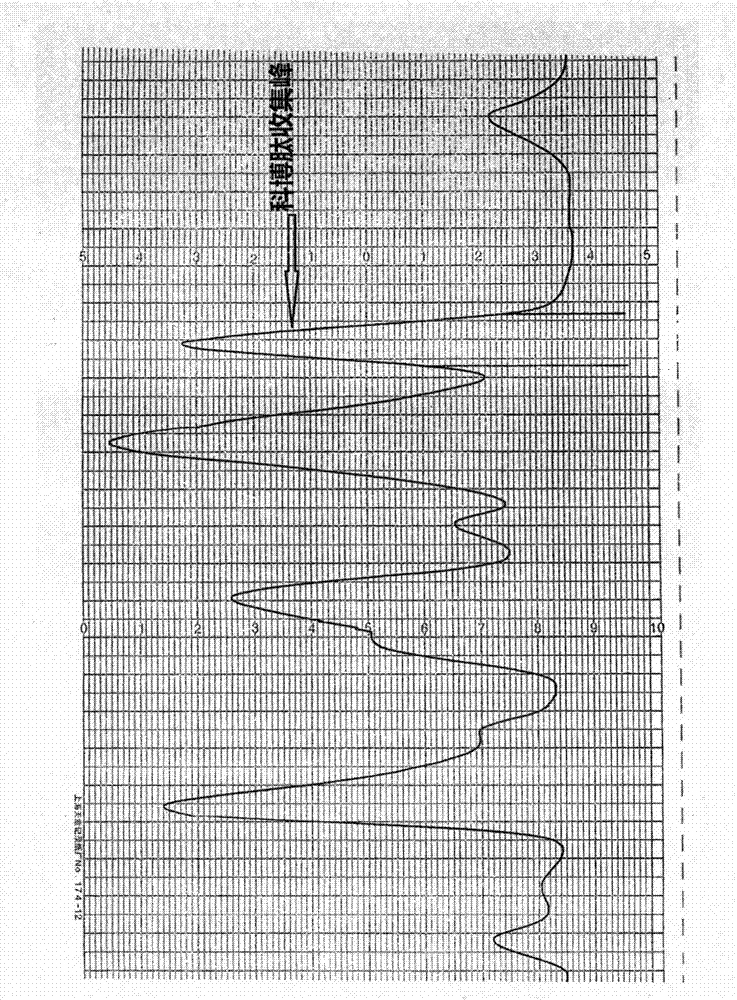
[0076] 2) Sp sepharose FF on the supernatant of snake venom, use PH 5.050mmol / L NaAC-HAC buffer with a salt gradient of 0.0-1.0mol / L NaCl for a total of 3000 ml of linear salt gradient elution, and the buffer to be eluted with a salt gradient When it reaches 0.7mol / L NaCl, collect the cobotide components according to the recorded spectrum, the cobotide neurotoxic protein peak is 320ml in total, see the chromatography results figure 1 ; Equipment setting parameters: ultraviolet detection parameters: 0.2A280nm; recorder parameters: 200mv 2cm / h; constant flow pump flow rate: 85ml / h; Low temperature dialysis at 4-10°C for 6 hours, wait for sample loading;
...
Embodiment 2
[0082] 1) Take 8 grams of crude cobra venom and dissolve it in 80 milliliters of 50 mmol / L Tris-HCl solution with pH 7.4. After fully dissolving, centrifuge at low temperature (temperature: 0°C--10°C, speed: 3600 rpm, time: 30 minutes), take the supernatant, and wait for the column;
[0083] 2) Sp sepharose FF on the supernatant of snake venom, use PH 7.4 50mmol / L Tris-HCl salt gradient buffer solution with a salt gradient of 0.0-1.0mol / L NaCl for a total of 3000 ml of linear salt gradient elution to collect Cobotide neurotoxic protein The total peak is 305ml; equipment setting parameters: UV detection parameters: 0.2A 280nm; recorder parameters: 200mv 2cm / h; constant flow pump flow rate: 85ml / h;
[0084] Subsequent steps are the same as in Example 1. Freeze-drying can obtain 378 mg of high-purity cobotide finished product, with a neurotoxic protein content of 78.20%; one SDS-PAGE electrophoresis is consistent with the cobotide reference substance; HPLC purity is 99.32%, and t...
Embodiment 3
[0086] 1) Take 8 grams of crude cobra venom and dissolve it in 80 milliliters of pH 6.850mmol / LTris-HCl solution. After fully dissolving, centrifuge at low temperature (temperature: 0°C--10°C, speed: 3600 rpm, time: 30 minutes), take the supernatant, and wait for the column;
[0087] 2) Sp sepharose FF on the supernatant of snake venom, use PH 6.8 50mmol / L Tris-HCl salt gradient buffer solution with a salt gradient of 0.0-1.0mol / L NaCl for a total of 3000 ml of linear salt gradient elution to collect Cobotide neurotoxic protein The total peak is 337ml; equipment setting parameters: UV detection parameters: 0.2A 280nm; recorder parameters: 200mv 2cm / h; constant flow pump flow rate: 85ml / h;
[0088] Subsequent steps are the same as in Example 1. Freeze-drying can obtain 362 mg of high-purity cobotide finished product, with a neurotoxic protein content of 80.35%; one SDS-PAGE electrophoresis is consistent with the cobotide reference substance; HPLC purity is 98.96%, and the chrom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com