Hybridoma cell and monoclonal antibody secreted by hybridoma cell, and applications
A hybridoma cell and monoclonal antibody technology, applied in the field of medical bioengineering, can solve the problems of long time, high cost, invasiveness of the human body, etc., and achieve the effects of stable cell chromosomes and high antibody titer.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1. Obtaining of hybridoma cell LCZ4H3 and the monoclonal antibody against prostasome protein produced by it.
[0024] 1. Extraction of prostasome protein
[0025]Take 500 ml of mid-morning urine from patients with chronic prostatitis, add an equal amount of HEPES buffer (pH7.3) and protease inhibitors (Sigma, USA), and filter with gauze. Store below -40°C for later use. Take the filtrate of the urine sample, place it in ten 50ml centrifuge tubes, and centrifuge at 400g for 10 minutes at a low temperature of 4°C. Remove the precipitate and take the supernatant. Centrifuge at 10,000g for 20 minutes at 4°C. Remove the precipitate and take the supernatant. Place them in 200 3ml ultracentrifuge tubes and centrifuge at 100,000g for 120 minutes at 4°C. Pour off the supernatant and take the precipitate. Add 2.5 ml of TBS buffer (pH7.8) and protease inhibitors (Sigma, USA), and mix well. Centrifuge at 100,000g for 120 minutes at 4°C. Pour off the supernatant and...
Embodiment 2
[0043] Example 2. The application of monoclonal antibody LCZ4H3 in the preparation of prostasome protein detection kit.
[0044] This kit uses enzyme-linked immunosorbent assay to quantitatively detect prostatic body protein (indirect ELISA method):
[0045] 1. Coat the prostasome protein diluted to a certain ratio in a 96-well plate (B1-F1 wells), add urine samples to the remaining wells, incubate in a 37°C incubator for 1 hour, wash the plate with a washing machine, add BSA to block, 100 microliters per well, incubated in a 37°C incubator for 40 minutes, and washed the plate 5 times. Add monoclonal detection antibody LCZ4H3, incubate for 40 minutes in a 37°C incubator, wash the plate, add HRP-labeled goat anti-mouse IgG, incubate in a 37°C incubator for 20 minutes, wash the plate and develop color in the dark, then add stop solution to stop reaction. The OD value of each reaction well at dual wave 450,630 nm was measured with a microplate reader. The standard curve is dr...
Embodiment 3
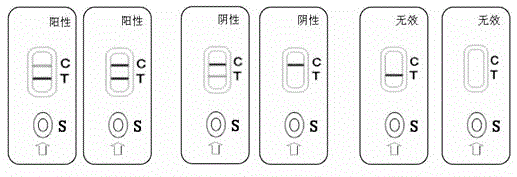
[0060] Example 3. Application of monoclonal antibody LCZ4H3 in the preparation of colloidal gold rapid detection test strips for detecting prostasome protein.
[0061] 1. It is made by adopting the principle of immunochromatography double-antibody sandwich method followed by colloidal gold labeling technology, and is used for the qualitative detection of prostasome protein in urine samples. Anti-prostasin antibody (T) is coated on the detection area of the reagent strip, and anti-mouse IgG antibody (C) is coated on the quality control area. During detection, the prostasome protein in the urine sample combines with the colloidal gold of the monoclonal antibody LCZ4H3 on the reagent strip to form a conjugate. The LCZ4H3 antibody reacted (T) with a red band. Regardless of whether there is prostasome protein in the specimen, when the liquid level continues to migrate to the fixed goat anti-mouse zone, a red band must appear in the quality control zone.
[0062] 2. If the col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com