Anti-CBirl hybridoma cell strain and monoclonal antibody produced by same
A hybridoma cell line and monoclonal antibody technology, applied in the field of immunology, can solve the problems of difficulty in obtaining serum or plasma, operator threat, raw material limitation, etc., and achieve the effects of chromosome stability, high titer, and avoidance of objective restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: CBir1 antigenic protein preparation
[0032] 1. Reagents and instruments
[0033] 1) Reagent
[0034] Escherichia coli host bacteria DH5α, BL21(DE3), cloning vector pCR2.1 T-vector, expression plasmid pET28a(+), DNA polymerase rTaq, T4 DNA ligase, DNA polymerase rTaq, LA Taq and restriction endonuclease BamH I, Hind III and EcoR I, BamH I, DL2000 DNA Marker, T4 DNA ligase, low molecular weight standard protein, DNA gel recovery kit, IPTG, etc.
[0035] 2) Instrument
[0036] Ordinary shaker SCS-24; water-proof constant temperature electric heating incubator; Biophotometer spectrophotometer, desktop refrigerated centrifuge Centrifuge 5810R, desktop centrifuge MiniSpin; high-speed refrigerated centrifuge; protein electrophoresis and gel imaging system; PCR instrument; ultrasound Lysis instrument; constant temperature metal bath; HIS protein purification column, etc.
[0037] 2. Experimental method
[0038] 1) Vector construction:
[0039] Design the pr...
Embodiment 2
[0044] Embodiment 2: the preparation of anti-CBir1 hybridoma
[0045] 1. Animal immunization
[0046] The purified CBir1 antigen was mixed with complete Freund's adjuvant in equal volume, emulsified with double syringes, and immunized 6-8 weeks old BALB / c mice by subcutaneous or intraperitoneal injection, with an immunization dose of 50 μg / mouse, with an interval of two weeks Afterwards, the second immunization was carried out, emulsified with incomplete Freund's adjuvant, and the immunization dose was 50 μg per mouse. After two times of immunization, the tail blood was taken to measure the serum titer by serial dilution by ELISA method; according to the results, it was determined whether to boost the immunization. Booster immunization was carried out 3 days before fusion, each mouse was intraperitoneally injected with 100 μg antigen without adjuvant, and cell fusion was carried out 3 days later.
[0047] 2. Cell Fusion
[0048] Pre-warm PEG1500 in a 37°C incubator before f...
Embodiment 3
[0054] Example 3: Preparation of anti-CBirl monoclonal antibody by hybridoma cells with deposit number CCTCC NO: C2016162
[0055] 1. Ascites Preparation
[0056] Male BALB / c mice aged 10-12 weeks were intraperitoneally injected with 0.5ml of liquid paraffin, and one week later, each mouse was intraperitoneally injected with monoclonal cell suspension washed and resuspended in PBS with a 1ml syringe, and the amount of cells used was 5×10 6 / Only. Ascites was collected after ascites accumulated in mice.
[0057] 2. Monoclonal Antibody Purification
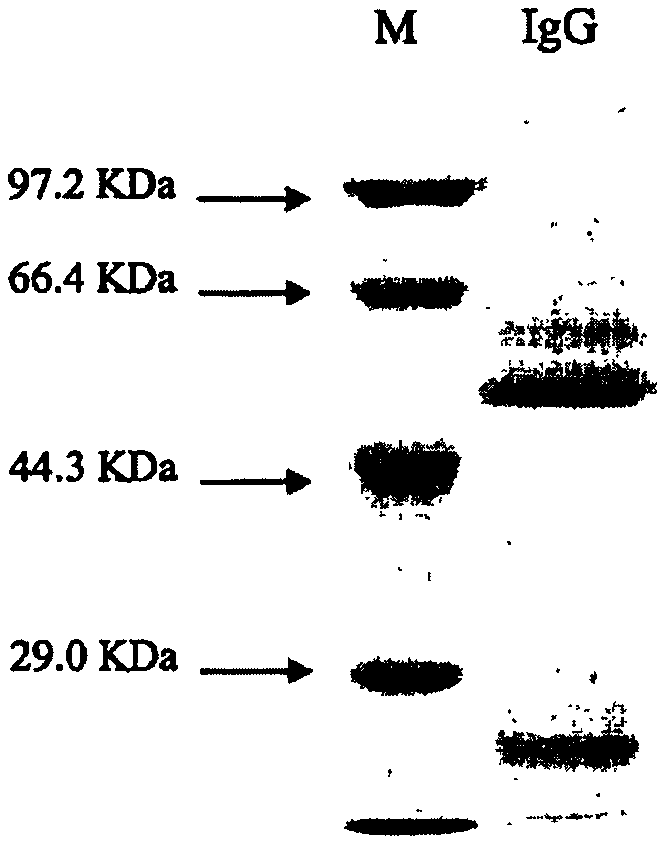
[0058] The ascites was centrifuged at 10,000 rpm for 10 minutes at 4°C to remove lipids. After centrifugation, the supernatant was aspirated and filtered with a 0.45 μm membrane. protein G was purified. The concentration of the purified monoclonal antibody was measured, subpackaged, and frozen at -20°C.
[0059] 3: ELISA method to identify monoclonal antibody subclasses
[0060] Dilute coated goat anti-mouse IgG (Zhongshan Ji...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com